Pharmacology Platform

INTRODUCTION
BOJIMED has established a dedicated technology service platform for drug efficacy and safety research, which holds NMPA GLP certification. The platform encompasses approximately 6,000 m² of animal laboratories and support facilities.
Our platform is structured into eight specialized departments—including Pharmacology, PK & Bioanalysis, Toxicology, Quality Assurance, and Laboratory Animal Services—and two committees, such as the Institutional Animal Care and Use Committee. Over 50% of our staff hold senior or intermediate positions.
Equipped with nearly RMB 30 million worth of advanced instruments—including physiological telemetry systems, BIOPAC recorders, animal ultrasound, laser Doppler flowmeters, blood gas analyzers, flow cytometers, fully automated analyzers, and LC-MS/MS systems—we possess the full capability to conduct comprehensive pharmacology, pharmacokinetics, and safety evaluations for chemical drugs, biologics, traditional Chinese medicines, and select medical devices.
Our platform is structured into eight specialized departments—including Pharmacology, PK & Bioanalysis, Toxicology, Quality Assurance, and Laboratory Animal Services—and two committees, such as the Institutional Animal Care and Use Committee. Over 50% of our staff hold senior or intermediate positions.
Equipped with nearly RMB 30 million worth of advanced instruments—including physiological telemetry systems, BIOPAC recorders, animal ultrasound, laser Doppler flowmeters, blood gas analyzers, flow cytometers, fully automated analyzers, and LC-MS/MS systems—we possess the full capability to conduct comprehensive pharmacology, pharmacokinetics, and safety evaluations for chemical drugs, biologics, traditional Chinese medicines, and select medical devices.
SCALE & EXPERIENCE
-
 500+
500+Pharmacology Studies
-
 200+
200+DMPK Studies
-
 500+
500+Toxicological and Safety Evaluation Project
-
 120+
120+Teams
-
 300+
300+Facilities
PHARMACOLOGICAL PLATFORM
Tumor Immunology Platform
CVD Platform
CNS Disease Platform
Respiratory Disease Platform
Endocrine and Metabolic Diseases Platform
TTDS Platform
TCM Syndrome Platform
Other Services
Tumor Immunology Platform
Diseases arising from the interplay between the immune system and tumors encompass a wide spectrum, including solid tumors (e.g., lung cancer, breast cancer, melanoma, colorectal cancer), hematologic malignancies (e.g., leukemia, lymphoma), and immune-related adverse events (e.g., checkpoint inhibitor-associated pneumonitis, colitis, cytokine release syndrome). These conditions involve the tumor microenvironment, immune escape mechanisms, and immune response regulation, forming the core focus of tumor immunotherapy research.
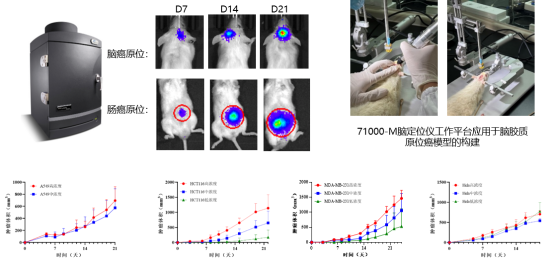
BOJIMED's Tumor Immunology Pharmacodynamic Evaluation Platform
In the ongoing pursuit of effective tumor immunotherapies, a precise and efficient efficacy evaluation system is essential for driving therapeutic breakthroughs. BOJIMED’s tumor immunology platform is built on multi‑dimensional technologies, providing reliable scientific support and decision‑making tools for drug development and efficacy assessment.
Core Strengths
Service
We are committed to providing innovative pharmaceutical companies, research institutions, and clinical centers with clinically predictive tumor immunology evaluation solutions. Through our multi‑dimensional technology platform and translational research system, we accelerate the development and implementation of next‑generation tumor immunotherapy strategies.
Case
CDX Tumor Model
BOJIMED's Tumor Immunology Pharmacodynamic Evaluation Platform
In the ongoing pursuit of effective tumor immunotherapies, a precise and efficient efficacy evaluation system is essential for driving therapeutic breakthroughs. BOJIMED’s tumor immunology platform is built on multi‑dimensional technologies, providing reliable scientific support and decision‑making tools for drug development and efficacy assessment.
Core Strengths
- Multi-Dimensional Evaluation System
Integrating immunological profiling (e.g., flow cytometry for immune cell subsets, ELISPOT for T‑cell responses), tumor biology assessment (e.g., tumor volume measurement, survival analysis, metastasis monitoring), molecular mechanism studies (e.g., RNA sequencing, Western blot for signaling pathways), and in vitro/in vivo model validation, we establish a comprehensive evaluation network from whole‑body efficacy to cellular and molecular mechanisms, capturing both drug effects and immune modulation dynamics.
- Precision Disease Models
|
Classification
|
Model
|
Modeling Method
|
|
Tumor Immunology
|
Xenograft
|
Human tumor cell lines or tissues transplanted into immunodeficient mice (e.g., nude, NSG) subcutaneously or orthotopically.
|
|
Syngeneic
|
Murine tumor cells implanted into immunocompetent syngeneic hosts (e.g., B16 melanoma in C57BL/6 mice).
|
|
|
GEMM
|
Transgenic, knock‑in, or conditional mutants (e.g., Cre‑LoxP) to activate oncogenes (e.g., KRAS) or inactivate tumor suppressors (e.g., p53).
|
|
|
Chemically Induced Models
|
Repeated exposure to carcinogens (e.g., DMBA/TPA for skin cancer, DEN for liver cancer).
|
|
|
Virus‑Induced Models
|
Introduction of tumor virus genes or infection (e.g., mouse papillomavirus models).
|
|
|
Spontaneous Tumor Models
|
Use of high‑incidence strains (e.g., AKR mice for leukemia, TRAMP mice for prostate cancer).
|
|
|
Metastasis Models
|
Tail vein injection (lung metastasis), intracardiac injection (bone metastasis), or monitoring of spread from orthotopic grafts (e.g., 4T1 breast cancer spontaneous metastasis).
|
|
|
PDX
|
Patient‑derived xenografts in immunodeficient mice reconstituted with human immune cells (e.g., CD34+ hematopoietic stem cells).
|
|
|
RA
|
CIA: Injection of type II collagen with adjuvant, K/BxN serum transfer model.
|
|
|
SLE
|
Spontaneous (e.g., NZB/W F1 mice) or induced models (e.g., pristane or anti‑DNA antibody injection).
|
|
|
Immunodeficiency Models
|
Gene‑knockout mice (e.g., Rag1⁻/⁻, SCID, Foxp3⁻/⁻) or humanized immune system reconstitution (e.g., CD34+ cells in NSG mice).
|
- Technology Highlights
1.Immune Cell Dynamic Monitoring System
Utilizes high‑dimensional flow cytometry and in vivo imaging to track infiltration, migration, and functional states of immune cells (e.g., CD8⁺ T cells, Tregs, macrophages) in the tumor microenvironment.
2.In Vivo Multi-Channel Immune Mircroenviornment PlatformI
Employs multiphoton microscopy and nanosensor technology for real‑time, in situ monitoring of immune synapse formation, cytokine secretion, and immune checkpoint dynamics.
3.Tumor Angiogenesis and Immune Cell Infiiltration Assessment
Integrates laser speckle contrast imaging and immunofluorescence 3D reconstruction to quantify spatiotemporal relationships between vascular normalization and immune cell infiltration efficiency.
4.Multi-Omic Biomarker Integration Analysis
Combines single‑cell sequencing, spatial transcriptomics, and multiplex protein detection to build predictive models for immunotherapy response and identify key biomarkers (e.g., PD‑L1, T‑cell clonality, IFN‑γ signatures).
Service
1. Efficacy Evaluation of Novel Immunotherapies
▷ Immune checkpoint inhibitors (anti‑PD‑1/PD‑L1, CTLA‑4 antibodies)
▷ Cell therapies (CAR‑T/TCR‑T, TIL therapies)
▷ Cancer vaccines (vaccines, DC)
▷ Bispecific antibodies and immune agonists
▷ Cell therapies (CAR‑T/TCR‑T, TIL therapies)
▷ Cancer vaccines (vaccines, DC)
▷ Bispecific antibodies and immune agonists
2. Mechanistic Studies and Target Validation
▷ Tumor immune escape mechanisms
▷ Immune cell exhaustion and memory differentiation regulation
▷ Functional validation and screening of novel immune targets
3. Combination Therapy Optimization
▷ Immunotherapy combined with radiotherapy/chemotherapy/targeted therapy/oncolytic viruses
▷ Strategies for managing immune‑related adverse events
▷ Research on reversing resistance mechanisms
▷ Strategies for managing immune‑related adverse events
▷ Research on reversing resistance mechanisms
4. Clinical Translation Support
▷ Development and validation of humanized tumor‑immune models (PDX‑HIS)
▷ Companion diagnostic biomarker development
▷ Bridging preclinical and clinical data for translational research
▷ Companion diagnostic biomarker development
▷ Bridging preclinical and clinical data for translational research
We are committed to providing innovative pharmaceutical companies, research institutions, and clinical centers with clinically predictive tumor immunology evaluation solutions. Through our multi‑dimensional technology platform and translational research system, we accelerate the development and implementation of next‑generation tumor immunotherapy strategies.
Case
CDX Tumor Model

CVD Platform
Diseases caused by structural or functional abnormalities of the cardiovascular and cerebrovascular systems encompass a wide spectrum. Common conditions include heart diseases (e.g., coronary artery diseases such as coronary heart disease and acute myocardial infarction; arrhythmias including atrial fibrillation and atrioventricular block; cardiac dysfunction such as heart failure; congenital heart diseases like atrial or ventricular septal defects; hypertension and related cardiac damage) and cerebrovascular diseases (e.g., ischemic stroke, hemorrhagic stroke such as intracerebral or subarachnoid hemorrhage, and transient ischemic attack). Other closely related disorders include peripheral vascular diseases (e.g., lower extremity arteriosclerosis obliterans, deep vein thrombosis), aortic diseases (e.g., aortic dissection, abdominal aortic aneurysm), and diabetic cardiomyopathy.
Cardiovascular Disease Pharmacodynamic Evaluation Platform
BOJIMED offers a comprehensive efficacy evaluation platform for cardiovascular and cerebrovascular diseases, integrating multimodal technologies to accelerate drug discovery and translation.
Core Strengths
Technology Highlights
Services
▶ Efficacy evaluation of novel drugs (small molecules, biologics, gene therapies)
▶ Mechanistic studies and target validation
▶ Drug repurposing and combination therapy screening
▶ Preclinical-to-clinical translational study design
We provide rigorous, standardized evaluation services to support researchers and innovators in advancing new therapies from bench to bedside.
Case
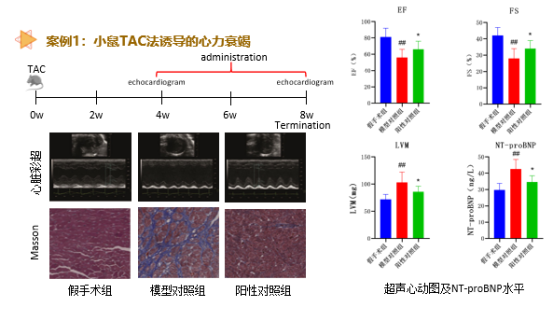
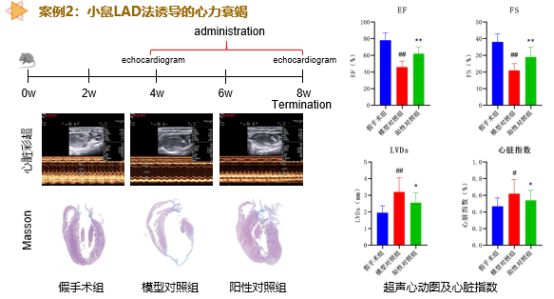
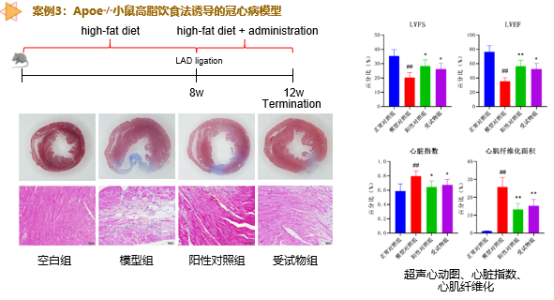
Cardiovascular Disease Pharmacodynamic Evaluation Platform
BOJIMED offers a comprehensive efficacy evaluation platform for cardiovascular and cerebrovascular diseases, integrating multimodal technologies to accelerate drug discovery and translation.
Core Strengths
- Multi-Dimensional Evaluation
Combines behavioral, imaging, histological, molecular, and biochemical analyses for holistic assessment from mechanism to outcome.
- Precision Disease Modeling
| Classification | Model | Method |
| cardiovascular system | heart failure | TAC/LAD |
| Doxorubicin induction | ||
| Abdominal aortic stenosis (AAC) | ||
| Ameroid narrowing ring implantation | ||
| Ang II. Induction (in vitro) | ||
| Myocardial ischemia-reperfusion | LAD reperfusion | |
| Acute myocardial ischemia | Isoproterenol-induced | |
| hypertension | SHR rats | |
| Dahl/SS rats | ||
| Induction between the two kidneys and a clamp | ||
| Vascular calcification | Vitamin D3 induction | |
| Chronic kidney disease vascular calcification | 5/6 nephrectomy + high phosphorus diet + adenine | |
| arrhythmia | Aconitine induction | |
| coronary heart disease | APOE gene knockout combined with high-fat diet modeling | |
| Coronary artery ligation (LAD) | ||
| High-fat diet combined with Pit induced coronary heart disease model | ||
| Coronal balloon injury method (small pigs) | ||
| Cerebral haemorrhage | Collagenase-induced rat model | |
| Autologous blood injection induced rats | ||
| MCAO+tPA model | ||
| Warfarin-induced rat model | ||
| Spontaneous hypertension model | ||
| Cardiomyopathy (dilated) | Coxsackievirus B3 (CVB3)-induced mouse model | |
| A rat model of long-term low-dose injection of doxorubicin | ||
| Cardiomyopathy (hypertrophic type) | Myocardium-specific MYH7 gene mutated mouse model | |
| Long-term isoproterenol subcutaneous injection induced rat model | ||
| Wild lily base (MCT)-induced rat model | ||
| Su5416 combined with hypoxia (SuHx)-induced mouse model | ||
| Hypoxia + MCT combined to induce severe illness | ||
| Aortic dissection | AngII. Continuous infusion of Apoe-/- mouse model | |
| Elastase perfusion rat aortic model | ||
| Acute pericarditis | Lipopolysaccharide (LPS) intraperitoneal injection induced rat model | |
| A rat model of xenoperitoneal injection of xenogeneic serum | ||
| stroke | MCAO induces ischemia-reperfusion stroke | |
| Hemorrhagic stroke induced by autologous blood or collagenase | ||
| atherosclerosis | HFD-induced ApoE-KO mouse atherosclerosis model |
Technology Highlights
▶ Imaging platform
▶ Cardiac catheterization technique
▶ Biomarker analysis
▶ Cardiac catheterization technique
▶ Biomarker analysis
Services
▶ Efficacy evaluation of novel drugs (small molecules, biologics, gene therapies)
▶ Mechanistic studies and target validation
▶ Drug repurposing and combination therapy screening
▶ Preclinical-to-clinical translational study design
We provide rigorous, standardized evaluation services to support researchers and innovators in advancing new therapies from bench to bedside.
Case



CNS Disease Platform
The human nervous system is prone to a diverse range of diseases, including cerebrovascular diseases (e.g., stroke), neurodegenerative disorders (e.g., Alzheimer's, Parkinson's), infectious diseases (e.g., meningitis), epilepsy, movement disorders, peripheral neuropathies, neuromuscular diseases, and headache disorders.
Other conditions involve demyelinating diseases, spinal cord injuries, autonomic dysfunction, sleep disorders, tumors, hereditary ataxia, neuropathic pain, and cognitive/psychiatric disorders.
CNS Pharmacodynamic Evaluation
A precise and efficient drug efficacy evaluation system is crucial for advancing neurological disease therapies.
Boji's platform leverages multi-dimensional technologies to provide scientific evidence and decision support for drug development.
Core Strengths
Technological Highlights
Services
We are committed to providing rigorous, efficient, and reproducible standardized evaluation services for universities, research institutions, and innovative pharmaceutical companies, accelerating the transition of clinically promising neurological therapies from the laboratory to clinical application.
Case
6-OHDA-Induced PD Model
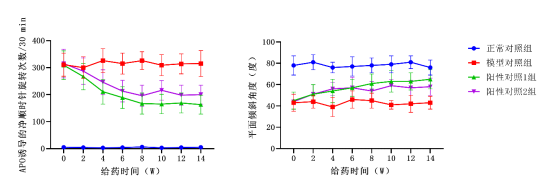
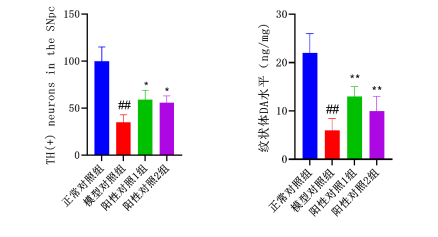
Other conditions involve demyelinating diseases, spinal cord injuries, autonomic dysfunction, sleep disorders, tumors, hereditary ataxia, neuropathic pain, and cognitive/psychiatric disorders.
CNS Pharmacodynamic Evaluation
A precise and efficient drug efficacy evaluation system is crucial for advancing neurological disease therapies.
Boji's platform leverages multi-dimensional technologies to provide scientific evidence and decision support for drug development.
Core Strengths
- Multi-Dimensional Evaluation
Combines behavioral analysis, electrophysiological recording, and molecular pathology to assess drug effects comprehensively, from behavior to mechanism.
- Accurate Disease Models
Utilizes validated rodent and non-human primate models for various neurological conditions (e.g., AD, PD, ALS, stroke), ensuring reliable research.
| Classification | Model | Method |
| nervous system | Parkinson's disease | MPTP induction, 6-OHDA induction, rotenone induction, proteasome inhibitor (e.g., Lactacystin) induction, α-synuclein virus vector injection |
| Alzheimer's disease | Transgenic animals (e.g., APP/PS1, 3xTg), β-amyloid induction, D-GaIN +AlCl3 induction, Tau protein model, lateral ventricular injection STZ | |
| vertigo | Uniform rotation induction, cervical vascular ligation induction, chloroform induction, vasopressin acetate induction, unilateral labyrinth destruction | |
| Neuropathic pain | Capsaicin induction, SNI (sparing nerve injury), SNL (spinal nerve ligation), PSNL (partial sciatic nerve ligation) | |
| Focal cerebral ischemia-reperfusion | Thrombus was prepared by wire thrombus (MCAO), electrocoagulation and photochemical methods | |
| Cerebral small vessel disease | Microemboli induction, bilateral common carotid artery stenosis (BCAS) method, NOS inhibitor (e.g., L-NAME) induction | |
| Focal cerebral infarction | Autologous thrombotic induction, electrocoagulation, photochemistry | |
| Cerebral haemorrhage | Collagenase VII.-heparin induction, autologous blood infusion method, microballoon filling method | |
| Cigarette addiction | Cigarette exposure induction, nicotine self-administration | |
| pain | Incision pain model, hot plate method, tail drifting method | |
| Inflammatory pain | CFA induction, formaldehyde induction, carrageenan induction, carrageenan induction | |
| Neuropathic pain | CCI method, sciatic nerve chronic compression injury (CCI), spinal nerve ligation (SNL) | |
| Diabetic pain | STZ-induced, streptozotocin-induced, db/db spontaneous diabetic mice | |
| Intestinal pain | Acetic acid torsion test, mustard oil induction, dextran sodium sulfate (DSS) induced colitis | |
| Multiple sclerosis | Experimental autoimmune encephalomyelitis (EAE), cuprizone (copper chelator) induces demyelination | |
| Amyotrophic lateral sclerosis | SOD1 transgenic mice, TDP-43 transgenic mice, FUS transgenic mice | |
| Huntington's disease | HTT transgenic mice, mitochondrial toxin (such as 3-NP)-induced, quinoline acid (QA) excitotoxic injury | |
| epilepsy | Pitetrazole (PTZ) ignition, sea acid (KA) induction, piruccapine induction, electroconvulsive ignition | |
| depression | Chronic Unpredictable Mild Stress (), Forced Swimming Experiment (FST), Tail Hanging Test (TST), Social Frustration Stress (SDS) | |
| Anxiety disorders | Elevated cross maze, open field experiment, light and dark box experiment | |
| schizophrenia | MK-801 (NMDA antagonist) induction, phencyclidine (PCP) induction, neonatal intraperitoneal injection poly I:C | |
| Post-traumatic stress disorder | Single prolonged stress (SPS), conditioned fear stress | |
| Spinal cord injury | Contusion injury (weight drop), transection, crush injury, photochemical injury | |
| Peripheral nerve damage | Sciatic nerve transect/ligation model | |
| Myasthenia gravis | Passive transfer model (injected with AChR antibody), active immune model (immunized animals with AChR) | |
| Brain tumors | In situ transplant models (C6, U87, GL261 cell lines), transgenic models (e.g., RCAS/tv-a systems) | |
| Attention deficit hyperactivity disorder | SHR (rats with spontaneous hypertension), 6-OHDA injection into the anterior cerebral cortex | |
| Drug addiction | Conditioned positional preference (CPP), behavioral sensitization |
Technological Highlights
▶ Behavioral monitoring system
▶ In vivo multi-channel electrophysiology platform
▶ Microvascular blood flow testing
▶ Biomarker analysis
▶ In vivo multi-channel electrophysiology platform
▶ Microvascular blood flow testing
▶ Biomarker analysis
Services
▶ Pharmacodynamic Evaluation of Innovative Drugs (Chemical Drugs, Biologics, Gene Therapy Products)
▶ Mechanism of Action Research and Target Validation
▶ Drug Repurposing and Combination Therapy Screening
▶ Preclinical-Clinical Translational Research Design
▶ Mechanism of Action Research and Target Validation
▶ Drug Repurposing and Combination Therapy Screening
▶ Preclinical-Clinical Translational Research Design
We are committed to providing rigorous, efficient, and reproducible standardized evaluation services for universities, research institutions, and innovative pharmaceutical companies, accelerating the transition of clinically promising neurological therapies from the laboratory to clinical application.
Case
6-OHDA-Induced PD Model


Respiratory Disease Platform
Respiratory diseases, as common and frequently occurring clinical conditions, have garnered significant research attention. Animal models are pivotal tools for exploring disease mechanisms, identifying potential therapeutic targets, and advancing drug development. Leveraging years of technical accumulation and research experience, BOJIMED's Pharmacology Research Department has established a rich array of respiratory disease models, encompassing IPF, asthma, acute pneumonia, bronchitis, pharyngitis, allergic rhinitis, cough, ALI, ARDS, COPD, PN, pulmonary edema, and PH.
Our Advantages
BOJIMED has successfully completed numerous respiratory drug efficacy evaluation studies, providing a reliable experimental platform for drug screening and efficacy assessment to pharmaceutical companies and research institutions. The platform enables comprehensive evaluation of pharmacodynamic indicators from functional, cellular, and molecular biology perspectives, offering diverse adaptability choices and reliable experimental support for preclinical research on respiratory innovation drugs.
Technical Highlights
1. Intelligent inhalation and drug administration
2. Accurate evaluation system
3. Personalized model design
4. Target organ toxicity assessment
5. Small animal in vivo imaging technology
Case
OVA-Induced Allergic Rhinitis Model
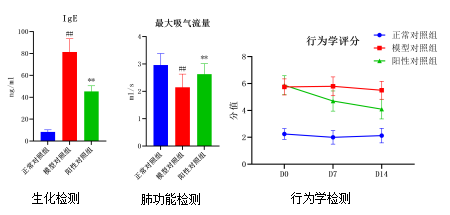
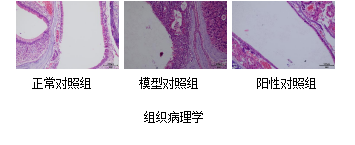
Our Advantages
BOJIMED has successfully completed numerous respiratory drug efficacy evaluation studies, providing a reliable experimental platform for drug screening and efficacy assessment to pharmaceutical companies and research institutions. The platform enables comprehensive evaluation of pharmacodynamic indicators from functional, cellular, and molecular biology perspectives, offering diverse adaptability choices and reliable experimental support for preclinical research on respiratory innovation drugs.
- Accurate Disease Models
| Classification | Disease | Method |
| Respiratory diseases | Idiopathic pulmonary fibrosis | Bleomycin-induced mouse model of pulmonary fibrosis |
| asthma | OVA-induced rat asthma model | |
| Al(OH)3+OVA induced asthma model in rats | ||
| allergic rhinitis | OVA peritoneal sensitization + nasal provocation induced rat and guinea pig models | |
| 2,4-toluene isocyanate (TDI) induced rat and guinea pig models | ||
| SO2+ estradiol benzoate induced rat model | ||
| Nasal net induction rat model | ||
| Bronchiectasis | Pseudomonas aeruginosa/Mycobacterium abscesses induced rat model | |
| Chronic obstructive pulmonary disease | Smoking + lipopolysaccharide induced COPD model | |
| Pulmonary edema | Ammonium chloride-induced pulmonary edema model | |
| Epinephrine-induced pulmonary edema model | ||
| LPS model of intratracheal instillation induced pulmonary edema | ||
| cough | Citric acid cough model of guinea pigs | |
| A model of asthma caused by histamine phosphoride in guinea pigs | ||
| Ammonia cough model in mice | ||
| Phenol red excretion test | ||
| pharyngitis | A model of acute pharyngitis caused by ammonia in mice | |
| Acute bronchitis | Smoking method and LPS-induced acute bronchial model |
Technical Highlights
1. Intelligent inhalation and drug administration
2. Accurate evaluation system
3. Personalized model design
4. Target organ toxicity assessment
5. Small animal in vivo imaging technology
Case
OVA-Induced Allergic Rhinitis Model


Endocrine and Metabolic Diseases Platform
Endocrine and metabolic diseases arise from functional abnormalities in endocrine glands or tissues, leading to excessive or insufficient hormone secretion.
This field encompasses DM, one of the four major chronic diseases, along with its various complications such as DKD, DPN, and diabetes mellitus complicated with DMED. It also includes UC, HU, GU, CLD, NASH, ACLF, OP, and HLP. Endocrine and metabolic disorders are not only core causes of diabetes but also significant risk factors and common grounds for cardiovascular diseases, malignancies, and other major chronic conditions. These diseases, typically complex and chronic, are interrelated, exhibit high incidence rates, long courses, are difficult to cure, and impose heavy medical burdens, being leading causes of death and disability worldwide. There exists a substantial unmet clinical need in this field, which presents a broad market prospect and has become a recent research hotspot for innovative drugs.
Pharmacodynamic Evaluation Platform
BOJIMED has successfully completed numerous respiratory drug efficacy evaluation studies, providing a reliable experimental platform for drug screening and efficacy assessment to pharmaceutical companies and research institutions. The platform enables comprehensive evaluation of pharmacodynamic indicators from functional, cellular, and molecular biology perspectives, offering diverse adaptability choices and reliable experimental support for preclinical research on respiratory innovation drugs.
Technical highlights and special services
1.Precision Metabolic Phenotyping Platform
Comprehensive in vivo metabolic monitoring: CLAMS integrated laboratory animal metabolic monitoring system (energy expenditure, respiratory quotient, activity levels).
High-precision blood glucose/insulin monitoring: Oral glucose tolerance test (OGTT), insulin tolerance test (ITT), hyperinsulinemic-euglycemic clamp technique (gold standard).
Molecular biology and omics integration: Tissue-level molecular detection (qPCR, WB, ELISA); metabolomics and lipidomics analysis based on mass spectrometry to reveal deep biomarkers and pathway changes; transcriptomics (RNA-seq) analysis for comprehensive drug mechanism parsing.
2.Advanced In Vivo Imaging Technology
micro-CT scanning for high-precision bone mineral density (BMD) and bone microstructure 3D analysis in osteoporosis models.
Liver pathology evaluation (NAFLD Activity Score, NAS) and collagen area quantitative analysis.
3.Gene Editing Model Customization
Utilizing CRISPR/Cas9 technology to rapidly construct desired gene knockout, point mutation, or humanized animal models.
We are dedicated to providing rigorous, efficient, and reproducible standardized evaluation services to universities, research institutions, and innovative pharmaceutical companies, accelerating the translation of innovative therapies for endocrine and metabolic diseases from the laboratory to patients.
Case
db/db Spontaneous DKD Mouse Model
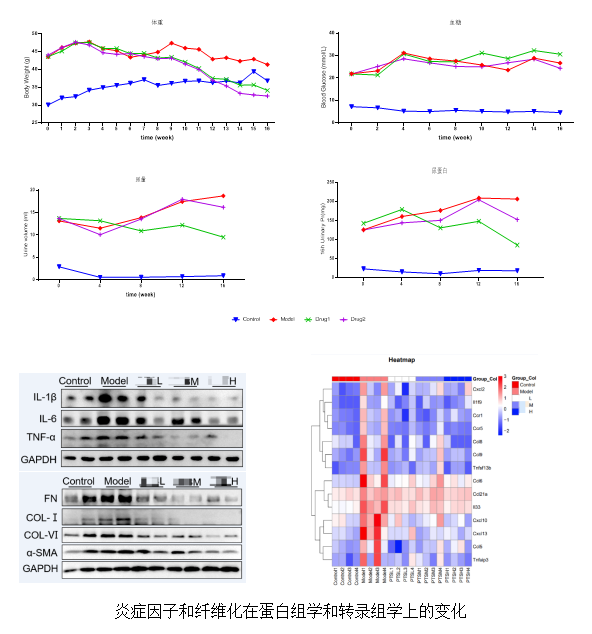
Pharmacodynamic Evaluation Platform
BOJIMED has successfully completed numerous respiratory drug efficacy evaluation studies, providing a reliable experimental platform for drug screening and efficacy assessment to pharmaceutical companies and research institutions. The platform enables comprehensive evaluation of pharmacodynamic indicators from functional, cellular, and molecular biology perspectives, offering diverse adaptability choices and reliable experimental support for preclinical research on respiratory innovation drugs.
- Disease Models
| Classification | Model | Method |
|---|---|---|
| endocrine and metabolic diseases | Type 2 diabetes | Dietary induction: High-fat diet (HFD) feeding Chemoinduction: Low-dose STZ injection Genetic engineering: db/db, ob/ob, ZDF rats Diet + chemical induction combination |
| Type 1 diabetes | Chemoinduction: high-dose STZ injection Spontaneous model: NOD mice |
|
| Diabetic nephropathy | db/db mice, STZ+ high-fat feeding (HFD). Mechanism exploration (in vitro): Glu/AGEs/inflammatory factors/antibodies |
|
| Diabetic foot ulcers | STZ + high-fat diet + full-thickness excision/incision | |
| Dietary obesity | DIO rats, mice | |
| Hereditary obesity | ob/ob 、db/db小鼠 | |
| Nonalcoholic steatohepatitis | Dietary induction: AMLN, HFD+STZ, high-fat, high-fructose-high-cholesterol (CDHFD) and other special feeds Chemical induction: CCl4 injection 基因工程: KK-Ay、MUP-uPA 小鼠 |
|
| Colon ulcers | Dextran sodium sulfate induction method (DSS), trinitrobenzene sulfonic acid induction method (TNBS), acetic acid induction method | |
| Cholestasis liver disease | 0.1% DDC feed feeding, bile duct ligation (BDL) | |
| Chronic acute liver failure | CCl4 combined with LPS/D-Gal N method, TAA combined with LPS method, serum albumin method | |
| Renal ischemia-reperfusion | Bilateral renal vein clamping and reperfusion | |
| Menopausal syndrome | Oophorectomy, geriatric, chemoinduction, genetically modified animals | |
| Primary dysmenorrhea | Estradiol + oxytocin induction | |
| Breast hyperplasia | Estradiol benzoate + progesterone induction | |
| osteoporosis | Bilateral ovariectomy, glucocorticoid induction, senile | |
| Hyperuricemia | Adenine/hypoxanthine induction, yeast paste/fructose/lipid emulsion induction, ethambutol/pyrazinamide/potassium oxazinate induction | |
| High blood lipids | High-fat/high-cholesterol/high-sugar diet induction method | |
| Atrophy of the genitourinary tract | ovariectomy | |
| Acute gout | Potassium oxazinate + hypoxanthine + sodium urate |
Technical highlights and special services
1.Precision Metabolic Phenotyping Platform
Comprehensive in vivo metabolic monitoring: CLAMS integrated laboratory animal metabolic monitoring system (energy expenditure, respiratory quotient, activity levels).
High-precision blood glucose/insulin monitoring: Oral glucose tolerance test (OGTT), insulin tolerance test (ITT), hyperinsulinemic-euglycemic clamp technique (gold standard).
Molecular biology and omics integration: Tissue-level molecular detection (qPCR, WB, ELISA); metabolomics and lipidomics analysis based on mass spectrometry to reveal deep biomarkers and pathway changes; transcriptomics (RNA-seq) analysis for comprehensive drug mechanism parsing.
2.Advanced In Vivo Imaging Technology
micro-CT scanning for high-precision bone mineral density (BMD) and bone microstructure 3D analysis in osteoporosis models.
Liver pathology evaluation (NAFLD Activity Score, NAS) and collagen area quantitative analysis.
3.Gene Editing Model Customization
Utilizing CRISPR/Cas9 technology to rapidly construct desired gene knockout, point mutation, or humanized animal models.
Services
▶ Pharmacodynamic evaluation of innovative chemical/biological/gene therapy products
▶ Mechanism of action research and target validation
▶ Screening of drug repurposing and combination drug regimens
▶ Preclinical to clinical translation research design
▶ Mechanism of action research and target validation
▶ Screening of drug repurposing and combination drug regimens
▶ Preclinical to clinical translation research design
We are dedicated to providing rigorous, efficient, and reproducible standardized evaluation services to universities, research institutions, and innovative pharmaceutical companies, accelerating the translation of innovative therapies for endocrine and metabolic diseases from the laboratory to patients.
Case
db/db Spontaneous DKD Mouse Model

TTDS Platform
Topical Drug Products (TDPs) are a class of drug formulations designed to act locally on the skin or systemically through skin penetration. Common dosage forms include ointments, creams, gels, and patches, primarily categorized into local skin TDPs and transdermal TDPs.
Pharmacodynamic Evaluation Platform
BOJIMED's Topical Drug Products pharmacodynamics evaluation platform specializes in the pharmacodynamic evaluation and technological development of topical skin drug delivery formulations (TDPs). Relying on GLP-certified laboratories, advanced experimental facilities, and a professional technical team, the platform has established various animal models for common skin diseases, including psoriasis and atopic dermatitis, providing scientific evidence for product optimization, aiding clinical value assessment, accelerating drug development, and promoting innovation in the TDP.
Comprehensive Pharmacodynamic Evaluation Services
Over 100 TDP pharmacodynamic evaluation services, covering mainstream dosage forms and indications.
Services
Case
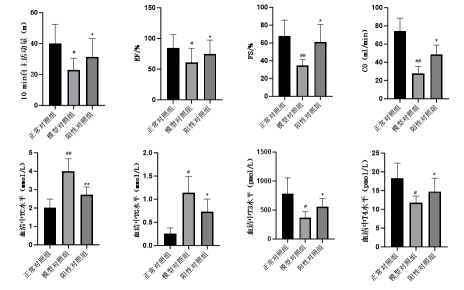
Psoriasis Model and Pharmacodynamic Evaluation for TDPs
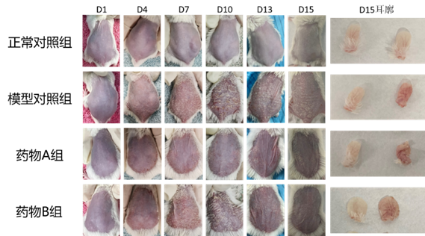
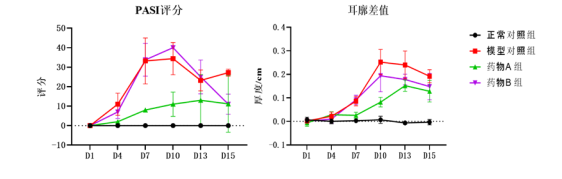
- Topical skin topical preparations mainly act on the skin or various layers of tissues under the skin, and their therapeutic areas include bacterial, fungal, viral infections, psoriasis, specific dermatitis, acne, etc.
- Transdermal preparations can enter the blood circulation system through the skin to exert systemic effects, and are often used for pain, central nervous system, cardiovascular system and other diseases. With the intensification of the global population aging and the increase in the number of chronic disease patients, the market demand for transdermal preparations continues to rise.
Pharmacodynamic Evaluation Platform
BOJIMED's Topical Drug Products pharmacodynamics evaluation platform specializes in the pharmacodynamic evaluation and technological development of topical skin drug delivery formulations (TDPs). Relying on GLP-certified laboratories, advanced experimental facilities, and a professional technical team, the platform has established various animal models for common skin diseases, including psoriasis and atopic dermatitis, providing scientific evidence for product optimization, aiding clinical value assessment, accelerating drug development, and promoting innovation in the TDP.
Comprehensive Pharmacodynamic Evaluation Services
- In Vitro Pharmacodynamic Screening: Evaluates drug mechanisms of action on skin targets using cell imaging and flow cytometry.
- In Vivo Pharmacodynamic Verification: Assesses local/systemic efficacy using animal models.
Over 100 TDP pharmacodynamic evaluation services, covering mainstream dosage forms and indications.
| Disease classify | Model | Method |
| skin disease |
Atopic dermatitis (AD). | DNCB excitation + OVA combined sensitization |
| eczema | DNCB, DNFB and other chemical reagents induce | |
| OVA antigen induction | ||
| Transgenic mouse model | ||
| Itching | Histamine+4-AP induction | |
| Allantoin-induced | ||
| P substance induction | ||
| Adenine induction | ||
| Deoxycholic acid induction | ||
| Histamine induction | ||
| skin cancer | UV induction | |
| Psoriasis (Ps). | imiquimod induction | |
| Hypertrophic scarring | Cutaneous perichondrial curettage induction | |
| Skin incision pain model | Linear full-thickness skin incision | |
| Pressure ulcers (PU). | Heavy object compression induction | |
| Scald model | Burn induction with a burn device | |
| Chronic skin ulcers | Skin lesions + Staphylococcus aureus + (glucocorticoids). | |
| Swelling of the anus | Croton oil induction |
Services
- Comprehensive In Vivo Pharmacodynamics Evaluation: Provides GLP-compliant in vivo pharmacodynamics studies using a mature skin disease model library (covering psoriasis, atopic dermatitis, acne, infectious skin diseases, etc.).
- Customized Services: Tailors animal models and experimental protocols based on client-specific needs, offering professional experimental design and optimization advice.
- Regulatory Submission Services: Provides comprehensive pharmacodynamic test reports and materials compliant with FDA, NMPA, and other regulatory requirements,
facilitating IND dual submissions and accelerating drug development and approval processes. Leveraging a professional technical team and extensive submission experience, we ensure the completeness and compliance of submission materials, enhancing submission success rates.
Case
Psoriasis Model and Pharmacodynamic Evaluation for TDPs


TCM Syndrome Platform
The human body may develop various TCM syndrome-related diseases due to yin-yang imbalance, visceral dysfunction, and qi-blood-fluid abnormalities.
Common syndromes include:
BOJIMED's preclinical platform integrates TCM theory with modern science to support R&D for TCM formulas, natural products, and integrated TCM-Western treatments.
Our Advantages
Technical Highlights
Services
We are committed to providing rigorous, efficient, and reproducible standardized evaluation services for universities, research institutions, and innovative pharmaceutical companies, accelerating the translation of clinically valuable new TCM drugs from the laboratory to the clinic.
Case
Coronary Heart Disease with Heart Yang Deficiency and Phlegm-Blood Stasis Syndrome Model
Common syndromes include:
- Qi Deficiency: Fatigue, shortness of breath
- Blood Deficiency: Pale complexion, palpitations
- Yin Deficiency: Five-center heat, night sweats
- Yang Deficiency: Aversion to cold, cold limbs
- Phlegm-Dampness: Obesity, excessive phlegm
- Damp-Heat: Bitter taste, sticky stools
- Qi Stagnation: Chest pain, frequent sighing
- Blood Stasis: Fixed stabbing pain, purplish tongue
- Visceral Syndromes: Liver yang hyperactivity (dizziness), heart-spleen deficiency (insomnia)
- Six-Meridian Syndromes: Taiyang wind stroke, shaoyang syndrome
- Wei-Qi-Ying-Xue Syndromes: Heat entering ying/blood levels
- Meridian Syndromes: Wind-cold-dampness arthralgia
- Emotional Disorders: Depression, hysteria
BOJIMED's preclinical platform integrates TCM theory with modern science to support R&D for TCM formulas, natural products, and integrated TCM-Western treatments.
Our Advantages
- Syndrome-Disease Models
Combines TCM syndromes (e.g., qi deficiency) with modern disease models (e.g., stroke) for "syndrome-disease-formula" evaluation
- Multidimensional Evaluation
Behavioral, physiological, biochemical, immunological, molecular, and pathological analyses
- Animal Models
| Classification | Model | Method |
| Symptoms of traditional Chinese medicine | Qi deficiency syndrome | Forced swimming depletion method, sleep deprivation method, food restriction method, high-load exercise training, hydrocortisone injection |
| Blood deficiency syndrome | Acetylphenylhydrazine (APH) combined with cyclophosphamide (CTX) injection, blood loss method, and chemical drugs (such as N-acetyl-p-benzoquinone) induction | |
| Yin deficiency syndrome | Thyroid hormone (such as levothyroxine) injection, hot traditional Chinese medicine (such as aconite, dried ginger) gavage, and high-heat environmental stimulation | |
| Yang deficiency syndrome | Hydrocortisone injections, adrenalectomy, gavage with thyroid depressants (such as prothiouracil), cold environmental stimulation | |
| Phlegm and dampness syndrome | High-fat feeding, humid environment (high humidity), fat and sweet diet (such as high sugar and high fat) combined with spleen deficiency modeling | |
| Blood stasis syndrome | Epinephrine injection superimposed on ice water stress, subcutaneous injection of epinephrine hydrochloride, polymer dextran injection, cold stimulation | |
| Liver depression syndrome | Chronic unpredictable mild stress (), restraint stress, clip-tail stress, social isolation method | |
| Spleen deficiency syndrome | Bitter cold diarrhea with traditional Chinese medicine (such as rhubarb and senna) by gavage, irregular diet (single day full + double day fasting), overwork (swimming), reserpine injection | |
| Kidney deficiency syndrome | Hydrocortisone injection, adenine feed feeding, aged naturally aging rats, ovary/orchiectomy (kidney yang deficiency/yin deficiency) | |
| Heart qi deficiency syndrome | Sleep deprivation combined with forced swimming, high-dose Xindean injection, and coronary artery ligation caused heart failure | |
| Spleen and stomach damp heat syndrome | High-fat and high-sugar diet + high temperature and high humidity environment + liquor gavage, endotoxin (LPS) injection superimposed on high temperature environment | |
| Liver depression and spleen deficiency syndrome | Chronic restraint stress + excessive swimming + eating disorders (triple method), combined with rhubarb gavage | |
| Kidney yang deficiency syndrome | Hydrocortisone injections, adenine feeding, elderly animals, surgical removal of the adrenal glands | |
| Kidney yin deficiency syndrome | Thyroid hormone injection, hot traditional Chinese medicine gavage, surgical removal of the thyroid gland | |
| Qi stagnation and blood stasis syndrome | Epinephrine injection superimposed on ice water stress, chronic restraint stress, subcutaneous injection of epinephrine hydrochloride | |
| Cold syndrome | Cold environment (such as 4°C freezer), frozen diet (such as ice water), cold traditional Chinese medicine (such as Coptis and Huangbai) for gavage | |
| Heat syndrome | Dried ginger, aconite and other hot traditional Chinese medicine gavage, endotoxin (LPS) injection, high temperature environmental stimulation | |
| Cardiovascular blood deficiency syndrome | Acetylphenylhydrazine (APH) injection, combined blood loss method (multiple blood sampling), cyclophosphamide injection | |
| Lung qi deficiency syndrome | Sulfur dioxide (SO₂) fumigation, cold stimulation superimposed vigorous swimming, papain intratracheal instillation | |
| Symptoms of instability of the defensive qi | Cold environment stimulation + fatigue swimming, hydrocortisone injection, cold wind stimulation | |
| Symptoms of heart deficiency and timidity | Empty bottle stimulation, sudden bright light/noise stress | |
| Hepatobiliary damp-heat syndrome | High-fat diet + alcohol gavage + endotoxin (LPS) or α-naphthalene isothiocyanate (ANIT) injections | |
| Cold and dampness trap the spleen | High humidity environment + cold environment + ice water gavage + high-fat diet | |
| Damp-heat injection syndrome | Escherichia coli perfusion method, high temperature and high humidity environment, and local injection of carrageenan | |
| Symptoms of hyperactivity in the heart | Thyroid hormone injections, chronic stress methods, alcohol/spicy stimulants by gavage | |
| Stomach heat syndrome | Alcohol gavage, capsaicin gavage, hot diet (such as dried ginger and aconite) feeding | |
| Intestinal dryness and fluid deficiency syndrome | Water restriction + dry diet (such as low fiber, high protein) + warm environment, loperamide hydrochloride gavage | |
| Rheumatic obstruction syndrome | Freund's complete adjuvant (CFA) joint injection, cold and humid environment stimulation, papain joint injection | |
| He did not transfer the certificate | Hormone (eg, estrogen/progesterone) cycle disorders, partial oophorectomy, social stress |
Technical Highlights
▶ Syndrome-disease model construction
▶ Serum pharmacology and drug analysis
▶ Multimodal biomarker integration
▶ Digital four-diagnostic analysis (tongue, posture, behavior)
▶ Serum pharmacology and drug analysis
▶ Multimodal biomarker integration
▶ Digital four-diagnostic analysis (tongue, posture, behavior)
Services
▶ Innovative TCM drug development
▶ TCM syndrome animal model evaluation
▶ Integrated TCM-Western treatment evaluation
▶ TCM syndrome animal model evaluation
▶ Integrated TCM-Western treatment evaluation
We are committed to providing rigorous, efficient, and reproducible standardized evaluation services for universities, research institutions, and innovative pharmaceutical companies, accelerating the translation of clinically valuable new TCM drugs from the laboratory to the clinic.
Case
Coronary Heart Disease with Heart Yang Deficiency and Phlegm-Blood Stasis Syndrome Model

Other Services
| Disease Classification | Animal Model | Modeling Methods |
|---|---|---|
| Digestive System Diseases & Metabolic Disorders | Ulcerative Colitis | DSS-induced, TNBS-induced, acetic acid-induced, IL-7 transgenic, TCRαKO |
| Chronic Superficial Gastritis | 60% ethanol + 20mM deoxycholate/bile + 0.05-0.1% ammonia + irregular feeding | |
| Chronic Atrophic Gastritis | Chronic Atrophic Gastritis | |
| Liver Cirrhosis | CCl4-induced, bile duct ligation | |
| Gastric Ulcer | Ice acetic acid-induced | |
| Duodenal Ulcer | Cysteamine hydrochloride-induced | |
| Viral Enteritis | Rotavirus-induced | |
| Cholelithiasis | Stone-inducing diet | |
| Hyperhomocysteinemia | Type 2 MTHFR gene knockout mice | |
| Hyperammonemia | Urease-induced | |
| Intestinal Obstruction | Small bowel interference induction (incomplete obstruction), loop ligation (adhesive incomplete obstruction), serosal stripping (acute/chronic) | |
| Acute/Chronic Alcoholic Liver Injury |
Alcohol/CCl4-induced | |
| Chronic Non-Alcoholic Fatty Liver Disease | :MCD diet-induced | |
| Acute Bile Stasis | Bile duct constriction-induced | |
| Chronic Liver Failure | CCl4 + D-GaIN/LPS-induced | |
| Skeletal System Diseases | Rheumatoid Arthritis | Collagen-induced, adjuvant-induced, collagen antibody-induced |
| Shoulder Periarthritis | Plaster fixation-induced left forelimb arthritis | |
| Knee Osteoarthritis | Medial meniscus + collateral ligament resection | |
| Acute Soft Tissue Injury | Soft tissue striker impact induction | |
| Infectious Diseases | Septic shock-induced lung injury | Lipopolysaccharide (LPS) induction |
| Radiation-induced proctitis | γ-ray induction | |
| acute ulcerative colitis | Salmonella induction | |
| Acute sinusitis | LPS induction | |
| Acute conjunctivitis | Croton oil induction | |
| Acute pharyngitis | Ammonia water induction | |
| Fever | Yeast induction | |
| Sensory System | Cataract | Aging-induced, sodium selenite induction, galactose induction |
| Glaucoma | Scleral vein cauterization, vitreous injection induction | |
| Dry eye syndrome | Scopolamine induction, benzalkonium chloride induction, lacrimal gland excision | |
| Allergic conjunctivitis | OVA induction, compound 48/80 induction | |
| Auricle swelling, acute nasal swelling |
Xylene induction | |
| Genitourinary System and Hormonal Disorders | IgA nephropathy | BSA combined with LPS induction; |
| Chronic renal failure | Cadmium (or iron) poisoning | |
| Renal ischemia-reperfusion | Bilateral renal vein occlusion followed by reperfusion | |
| Vaginitis | Staphylococcus aureus induction |