INTRODUCTION
The treatment of infectious diseases represents a critical area in modern medicine, encompassing various viral conditions such as hepatitis (particularly hepatitis B and C), human immunodeficiency virus (HIV) infection, and human papillomavirus (HPV) infection. With the acceleration of globalization, the transmission speed and scope of viral diseases have also increased, particularly hepatitis B and C. Long-term chronic infection can lead to severe liver damage, including cirrhosis and liver cancer. Due to rapid viral mutation, treatment challenges, and poor response to existing therapies in some patients, these diseases pose significant public health challenges. Effective antiviral drugs not only improve patients' quality of life but also effectively control disease transmission.
PROJECT EXPERIENCE

Recombinant Human Albumin, a Class 1 Biological Product for Therapeutic Use, Successfully Launched
Boji Pharmaceutical provided Phase III clinical research services for this project, coordinating, supervising, and managing the entire clinical research process. In accordance with regulatory review requirements, Boji submitted the corresponding clinical trial summary report and a complete set of application materials (including but not limited to ethics review reports, case report forms/EDC, data management reports, statistical analysis reports, and summary reports). This demonstrates Boji Pharmaceutical's exceptional capabilities in delivering comprehensive, one-stop drug research services.
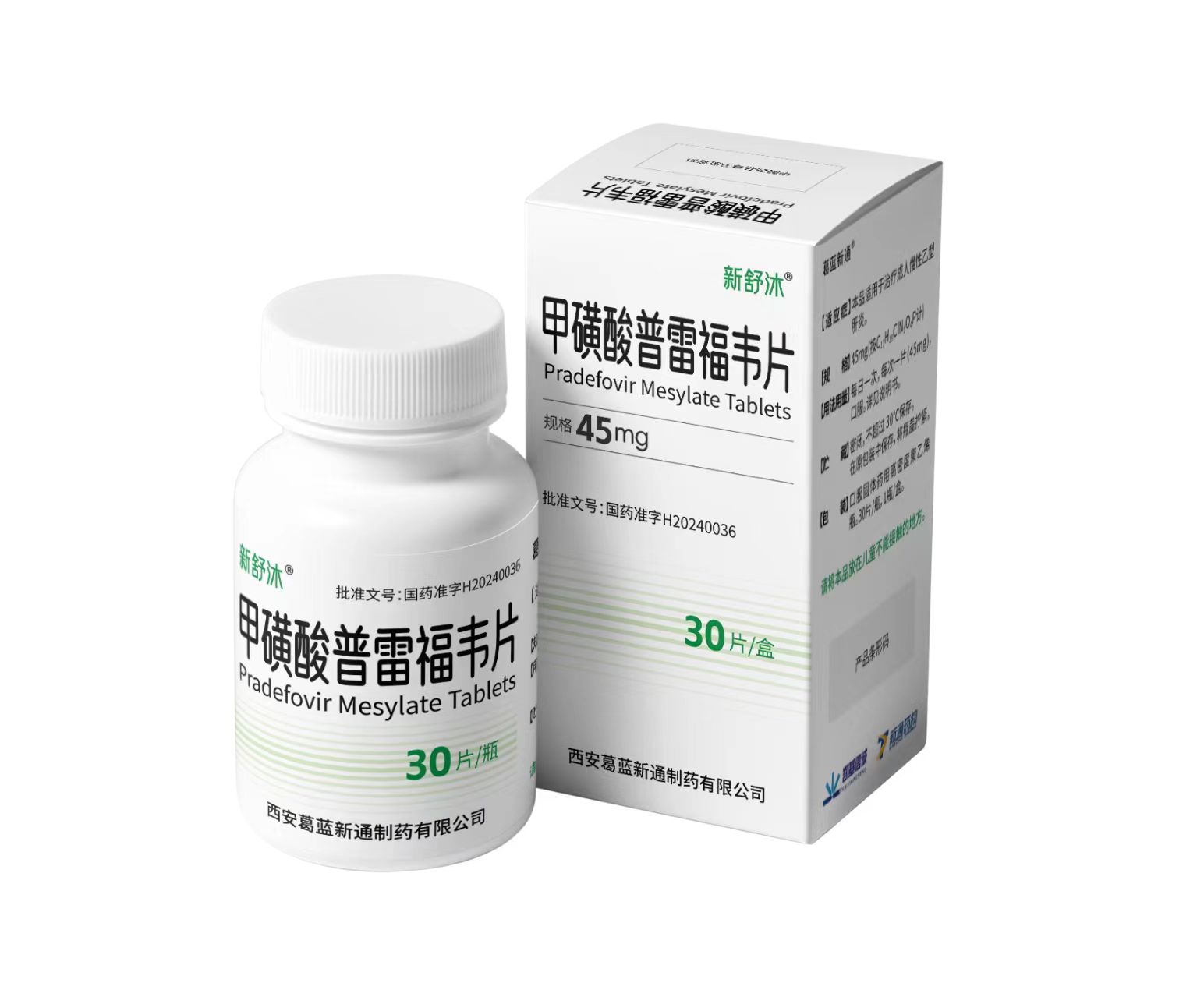
Assisting in the successful approval and market launch of a Class 1 innovative hepatitis B drug
As the CRO for the clinical research of Prefovir Disoproxil Fumarate Tablets, Boji Pharmaceutical provided comprehensive support throughout the clinical trial process and participated in communications with the CDE. The project enrolled over 1,000 patients across more than 60 centers nationwide, completing all subject recruitment in under 10 months. This demonstrates Boji Pharmaceutical's exceptional R&D service capabilities and high-quality, efficient clinical operations in organizing multi-center, large-sample, and high-complexity clinical trials for innovative drugs.
Assisting in the Approval and Market Launch of a Class 1 COVID-19 Treatment Drug
Boji Pharmaceutical leveraged its extensive experience and professional team to provide scientific and rigorous management services for the clinical trial of the Atorvastatin/Ritonavir combination packaging project, ensuring the trial's smooth execution.

Assisting in the approval and market launch of a new Class 1 anti-infective drug
Leveraging extensive experience and a specialized team, Boji Pharmaceutical provided scientific and rigorous management services for the clinical trials of the cantizolam tablets project, ensuring the trials' successful execution.
Supporting Post-Marketing Studies for Human Interferon G
Leveraging our expertise in post-marketing research for biological products, our team conducted high-quality data collection and analysis. This provided crucial evidence for the product's rational clinical application and safety evaluation, supporting the client in enhancing its product lifecycle management. This fully demonstrates our professional service capabilities and technical strengths in post-marketing research for biological drugs.

Proficient in the full spectrum of hepatology treatment and drug development services