About Boji
About Us
Boji Medical Technology Co., Ltd.(referred to as “BojiMed,” stock code: 300404) was established in 2002 and listed on the Shenzhen ChiNext Board in 2015. With a registered capital of RMB 382 million, it is a new high-tech enterprise providing comprehensive “one-stop” outsourcing services (CRO+CDMO) for drug and medical device R&D and manufacturing to domestic and international pharmaceutical companies.
The company operates from 120,000 square meters of modern office, laboratory, and production facilities, currently employing over 1,400 staff. It owns more than 30 wholly-owned and controlled subsidiaries, along with over a dozen equity-participated companies engaged in related businesses. It has earned numerous honors including: Top 10 Pharmaceutical Outsourcing Companies in China, Top 10 Most Competitive Pharmaceutical Service Enterprises in China, National Specialized, Sophisticated, Distinctive, and Innovative “Little Giant” Enterprise, Guangdong Provincial Enterprise Technology Center, Guangdong Provincial Postdoctoral Innovation Practice Base, China's Top 50 Most Investment-Worthy Enterprises, and Honorary Chair Unit of the CRO Branch of the China Pharmaceutical Quality Management Association. As a leading full-service CRO in China, it is also the first publicly listed CRO primarily focused on clinical trials.
BojiMed's “one-stop” services encompass: new drug project initiation and active compound screening; pharmaceutical research (API, formulations); drug evaluation (pharmacodynamics, toxicology); integrated small molecule innovation drug services; clinical research (including medical devices); dual China-US drug/device registration services (regulatory submissions); CDMO manufacturing (MAH implementation); and technology commercialization—covering all stages of new drug development.
Contact Number: 020-38473208
Collaboration Email: bd03@gzboji.com
The company operates from 120,000 square meters of modern office, laboratory, and production facilities, currently employing over 1,400 staff. It owns more than 30 wholly-owned and controlled subsidiaries, along with over a dozen equity-participated companies engaged in related businesses. It has earned numerous honors including: Top 10 Pharmaceutical Outsourcing Companies in China, Top 10 Most Competitive Pharmaceutical Service Enterprises in China, National Specialized, Sophisticated, Distinctive, and Innovative “Little Giant” Enterprise, Guangdong Provincial Enterprise Technology Center, Guangdong Provincial Postdoctoral Innovation Practice Base, China's Top 50 Most Investment-Worthy Enterprises, and Honorary Chair Unit of the CRO Branch of the China Pharmaceutical Quality Management Association. As a leading full-service CRO in China, it is also the first publicly listed CRO primarily focused on clinical trials.
BojiMed's “one-stop” services encompass: new drug project initiation and active compound screening; pharmaceutical research (API, formulations); drug evaluation (pharmacodynamics, toxicology); integrated small molecule innovation drug services; clinical research (including medical devices); dual China-US drug/device registration services (regulatory submissions); CDMO manufacturing (MAH implementation); and technology commercialization—covering all stages of new drug development.
Contact Number: 020-38473208
Collaboration Email: bd03@gzboji.com

创新药械一站式
全流程CRO+CDMO服务商
Development History
Our Advantage




Highly experienced
BOJIMED has been deeply rooted in the CRO industry for 23 years and celebrated its 10th anniversary as a listed company on the A-share market. As one of China's earliest CRO service providers, it boasts extensive project experience, concentrated industry resources, precise service solutions, and a comprehensive quality system.
Quality First
Since its establishment, BOJIMED has successfully completed all clinical projects reviewed by the National Medical Products Administration. The company maintains seamless coordination across all departments, prioritizes quality, ensures efficient communication, and guarantees project timelines.
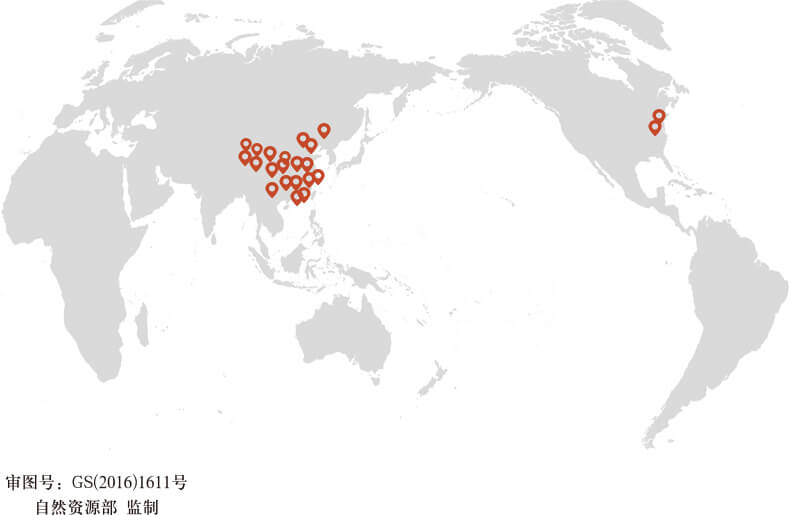
Global footprint
BOJIMED has established a global presence with subsidiaries in mainland China, Hong Kong, China, and Maryland, USA. We are well-versed in regulatory requirements for registration and clinical trials in Europe, the Americas, and Asia-Pacific countries, enabling us to provide comprehensive global clinical services.
Team Strengths
BOJIMED boasts numerous experts with national-level regulatory review backgrounds. Its core team maintains direct communication channels with the FDA, CDE, and leading principal investigators in the industry, fostering strong collaborative relationships that enable project advancement across multiple levels. With a dedicated Study Start-Up team of nearly 100 professionals, the company demonstrates significant advantages in driving preliminary launch activities—including site selection, site processes, and site initiation—ensuring seamless integration between regulatory and clinical work to accelerate product clinical development timelines.