Drug Research & Lab Testing

INTRODUCTION
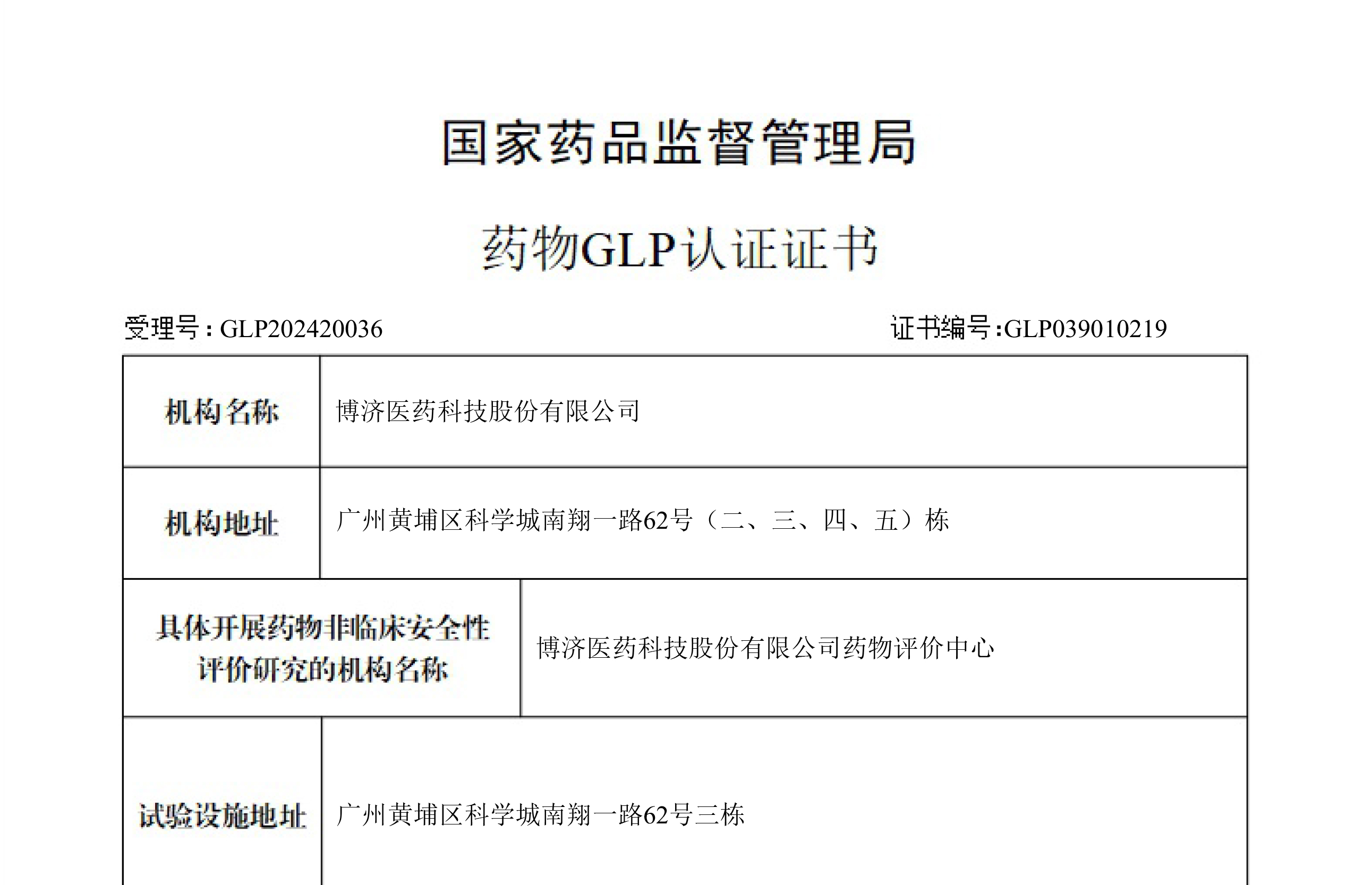
BOJIMED has established a dedicated technology service platform for drug efficacy and safety research, which holds NMPA GLP certification. The platform encompasses approximately 6,000 m² of animal laboratories and support facilities.
Our platform is structured into eight specialized departments—including Pharmacology, PK & Bioanalysis, Toxicology, Quality Assurance, and Laboratory Animal Services—and two committees, such as the Institutional Animal Care and Use Committee. Over 50% of our staff hold senior or intermediate positions.
Equipped with nearly RMB 30 million worth of advanced instruments—including physiological telemetry systems, BIOPAC recorders, animal ultrasound, laser Doppler flowmeters, blood gas analyzers, flow cytometers, fully automated analyzers, and LC-MS/MS systems—we possess the full capability to conduct comprehensive pharmacology, pharmacokinetics, and safety evaluations for chemical drugs, biologics, traditional Chinese medicines, and select medical devices.
Our platform is structured into eight specialized departments—including Pharmacology, PK & Bioanalysis, Toxicology, Quality Assurance, and Laboratory Animal Services—and two committees, such as the Institutional Animal Care and Use Committee. Over 50% of our staff hold senior or intermediate positions.
Equipped with nearly RMB 30 million worth of advanced instruments—including physiological telemetry systems, BIOPAC recorders, animal ultrasound, laser Doppler flowmeters, blood gas analyzers, flow cytometers, fully automated analyzers, and LC-MS/MS systems—we possess the full capability to conduct comprehensive pharmacology, pharmacokinetics, and safety evaluations for chemical drugs, biologics, traditional Chinese medicines, and select medical devices.
SCALE & EXPERIENCE
-
 500+
500+Pharmacology Studies
-
 200+
200+DMPK Studies
-
 500+
500+Toxicological and Safety Evaluation Project
-
 120+
120+Teams
-
 300+
300+Facilities