INTRODUCTION
Our platform is structured into eight specialized departments—including Pharmacology, PK & Bioanalysis, Toxicology, Quality Assurance, and Laboratory Animal Services—and two committees, such as the Institutional Animal Care and Use Committee. Over 50% of our staff hold senior or intermediate positions.
Equipped with nearly RMB 30 million worth of advanced instruments—including physiological telemetry systems, BIOPAC recorders, animal ultrasound, laser Doppler flowmeters, blood gas analyzers, flow cytometers, fully automated analyzers, and LC-MS/MS systems—we possess the full capability to conduct comprehensive pharmacology, pharmacokinetics, and safety evaluations for chemical drugs, biologics, traditional Chinese medicines, and select medical devices.
SCALE & EXPERIENCE
-
 500+
500+Pharmacology Studies
-
 200+
200+DMPK Studies
-
 500+
500+Toxicological and Safety Evaluation Project
-
 120+
120+Teams
-
 300+
300+Facilities

Core Services
SERVICE FEATURES
Transdermal and Topical Formulation Development
-
Dosage Forms: Patches, gel plasters, ointments, creams, liniments, films.
-
Animal Models: Rats, guinea pigs, rabbits, minipigs.
-
Indications: Rheumatoid arthritis, sprains, burns, diabetic neuropathy.
-
Endpoints: Local irritation, toxicokinetics, efficacy.
-
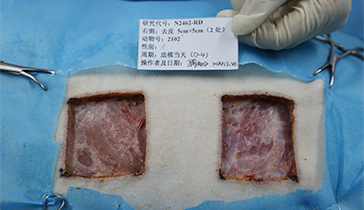
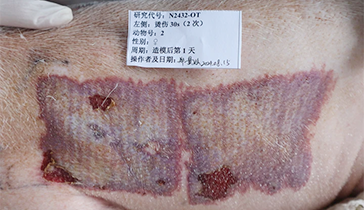
Wound Models: Deep partial-thickness burns, excised skin, impaired skin.

Inhalation Formulation Development
-
Dosage Forms: MDIs, nebulizers, DPIs, liquids.
-
Delivery Methods: Intranasal/tracheal instillation, intratracheal aerosolization, oronasal dynamic inhalation.
-
Aerosol Monitoring: Particle size distribution, concentration, formulation analysis.
-
Respiratory Evaluation: Lung function, histopathology, mucociliary clearance, BALF analysis.
-
Tissue Distribution: Local exposure, plasma levels, tissue analysis.



Pediatric Drug Development
-
Physical Growth
-
Skeletal Development
-
Cardiovascular/Respiratory Function
-
Neurobehavioral Development
-
Sexual Maturation
-
Immune Function



ATMPs
-
Cell Therapy (genetically modified/unmodified)
-
Gene Therapy (nucleic acids, viral vectors, gene editing)
-
Other Advanced Modalities (tissue-engineered, neoantigen, novel delivery systems, cell derivatives)