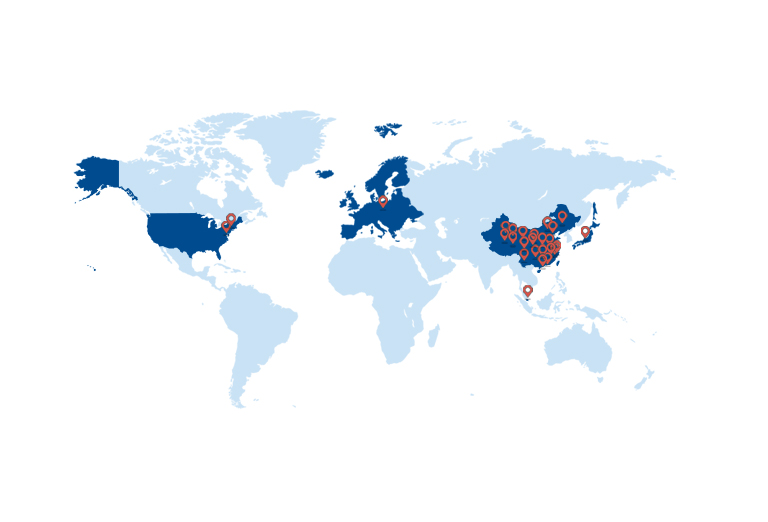
Global Registration Applications
INTRODUCTION
INTRODUCTION
Bo Ji Pharmaceutical | Global Registration Strength — "Three-in-One" System, Professional Teams & US Subsidiary, Empowering Clients with Compliant & Efficient Global Drug Registration.
OUR SCALE
-
 60+
60+Communication Projects
-
 70+
70+Domestic IND Application Projects
-
 40+
40+Marketing Authorization Application (MAA) Projects (including ANDA, NDA and BLA)
-
 100+
100+FDA Investigational New Drug (IND) Application Projects
OUR ADVANTAGES
SERVICE CONTENT

Chemical Drug Registration Services
- NMPA:New/Generic Drugs Clinical Application (IND), New Drug Application (NDA) & Generic Drug Application (ANDA), Filing of APIs, Excipients and Packaging Materials, Bioequivalence (BE) Filing, etc.
- FDA:505 (b)(1) & 505 (b)(2) Pathway Filings, Abbreviated New Drug Application (ANDA), Orphan Drug Designation (ODD), DMF Registration for APIs, Excipients and Packaging Materials, etc.
- EMA:Scientific Advice, Clinical Trial Application (CTA), Generic Drug Marketing Authorization Application (MAA), CEP/ASMF Applications for APIs, Excipients and Packaging Materials, etc.

Biological Products Registration Services
- NMPA:IND and NDA/BLA for Therapeutic and Preventive Biological Products (including antibodies, fusion proteins, Cell and Gene Therapy (CGT) products, etc.)
- FDA:Biologics IND, Orphan Drug Designation (ODD), Fast Track Designation (FTD) – formerly referenced as RPDD, and other strategic filings

Traditional Chinese Medicine
- China:Innovative Traditional Chinese Medicine (TCM) Drugs, TCM Drugs with the Same Name and Same Formula, Classical TCM Formulas, TCM Institutional Preparations, Protected TCM Varieties, Over-the-Counter (OTC) Conversion;
- International:TCM Registration in Hong Kong/Macao Special Administrative Region (SAR), US FDA Botanical Drug Investigational New Drug (IND) Application