

























For more questions, please click "Consult Now", a professional consultant will answer your questions.








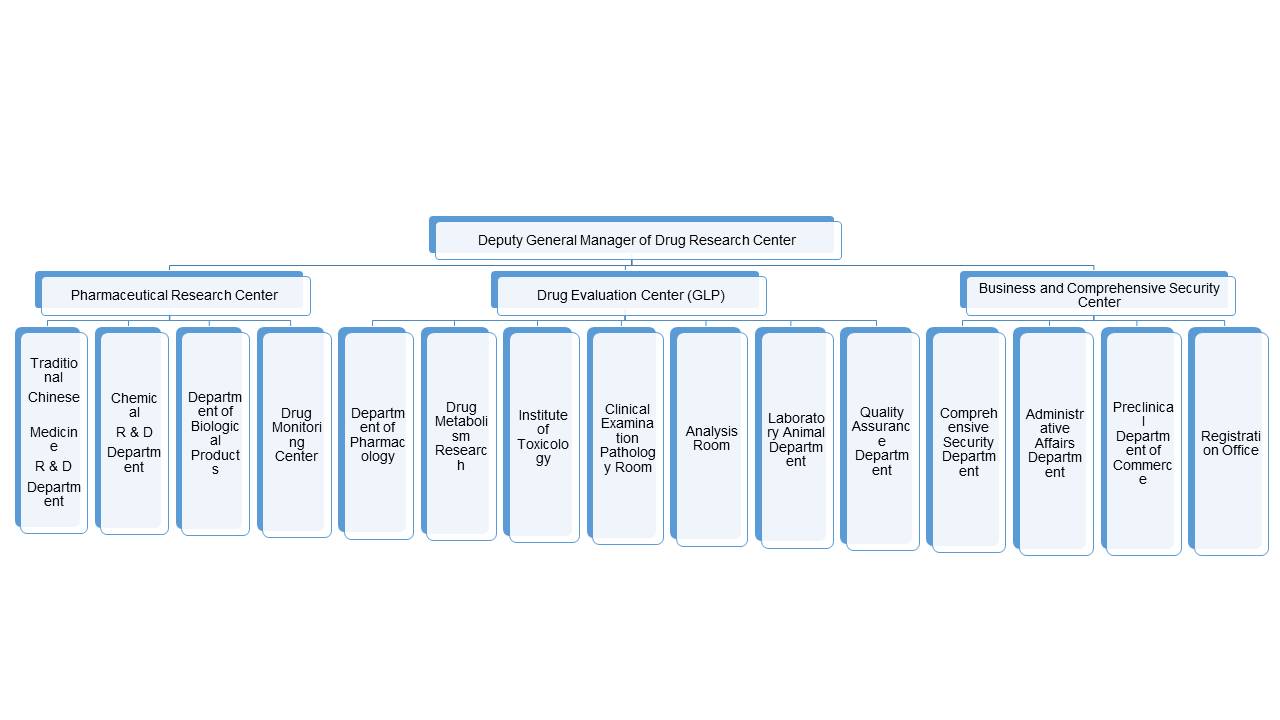
Boji Pharmaceutical Research Center("Center" for short) is a specialized institution that provides pharmaceutical research services such as API, preparation technology and quality standard study. With more than 10-year development, the center has a strong technical strength. Not only can we develop generic drugs and improved innovative medicine, but also independently develop Class 1 innovative drugs for customers. By the end of 2019, the center had conducted more than 100 new drugs development and technical service from customers. At the same time, it had achieved milestones in the transfer of independent development projects, such as the new class 1 drug Ginkgolide B and its injection, the new class 2 chemical drug gatifloxacin ear drops, pazufloxacin mesylate ear drops, Chinese medicine Xinmaikang capsules, Huoluotongnao tablets, Bingliancao tablets, etc. They have all obtained IND approvals. Class 1 new medicines HYA, ASP, CRN, HCF and HEDS are yet to be transferred.
|
|
|
|
Extraction Pilot Workshop for Chinese Medicine | Plan of Pilot Workshop for Preparation | Pilot Cleaning Workshop for Biological Product | Quality Inspection and Analysis Laboratory |
1、Traditional Chinese Medicine R & D Department
The department is under the responsibility of the director and controlled by the research room. There are currently more than 20 professional and technical personnel. It has a small-scale research, a pilot-scale extraction workshop, and a quality research laboratory. Equipped with more than 10 million rmb worth of scientific research equipment, it has more than 1,000 square meters of experimental space. We have carried outstandardized research and application in the field of many technologies, and formed a series of professional technologies, including Key technologies such as ultrafine crushing, multifunctional extraction, membrane separation, medium pressure chromatography, preparative chromatography, finger prints of traditional Chinese medicine, extract drying, moisture resistance. We can conduct research and production of oral solid preparations, external preparations, and injections in Traditional Chinese Medicine and natural medicines.
|
|
|
|
Traditional Chinese Medicine Process Research Office | Raw Material Refining Workshop (Purification Equipment) | Gas-Atomic Absorption Laboratory | Vacuum and Spray Drying Room |
2、Chemical R & D Department
The department is under the responsibility of the director and controlled by the research room. There are departments such as synthesis research room, preparation research room, quality research room, synthesis pilot workshop and preparation pilot clean workshop. It has more than 2,000 square meters of experimental space, equipped with more than 20 million rmb of scientific research and production equipment. This department can carry out research on the development of innovative drugs, generic drugs, consistency evaluation technology and quality standards.
|
|
|
|
Preparation Pilot Plant (Automatic Capsule Filling Machine, etc.) | Chemical Quality Analysis Room | Chemical Synthesis Room | API Pilot Scale-up |
3、Biological Products R & D Department
The Biological Product Research and Development consists of fermentation room, purification room and quality testing room. The main technical experts have more than 10 years of experience in protein and fermentation research, mainly engaging in recombinant expression, animal tissue extraction and purification research, research and development of cosmetic peptides, peptides and antibodies, fermentation process research, analysis methods and pilot production.
|
|
|
|
Amino Acid Analyzer | High-pressure Cell Disruption Instrument | 140L Biological Fermentation Tank | Preparative Liquid Chromatograph |
4、Drug Analysis Center
In order to ensure the quality of medicine research, the company established a drug analysis center in 2016 and a medicine research quality assurance system. In July 2018, the center passed the on-site assessment of the China National Accreditation Service for Conformity Assessment (CNAS), obtained a laboratory accreditation certificate and has more than 16 drug testing capabilities. The center has professional drug analysis capabilities.
The center currently consists of more than 20 experienced professional and technical personnel who have participated inprofessional technical trainings. The center is a well-organized, professional, high-quality team equipped with multiple high-performance liquid chromatographies, gas chromatographs, automatic sampling dissolution apparatus, UV-visible spectro photometers, infrared spectro photometers and other advanced instruments. The center can provide accurate data analysis reports.
Technical service content
1. Research and development of innovative drugs and improved new drugs
(1) Research and development of innovative chemical drugs
(2) Research and development of traditional Chinese medicine and natural medicine
(3) Research and development of biological products for treatment and prevention
2. Generic drug research and drug re-evaluation
(1) Development of class 3 or 4 generic drugs (including solid preparations, inhalation preparations, and injections)
(2) Consistency evaluation study of oral solid preparation drugs
(3) Re-evaluation of traditional Chinese medicine and chemical injections
3. Pharmaceutical research technology services
(1) Technical services development for Chinese medicine, natural medicine and chemical medicine
(2) Research on the process technology and quality of biological products
(3) Pilot research and sample manufacture simulators
(4) R & D and production of clinical simulators and placebos
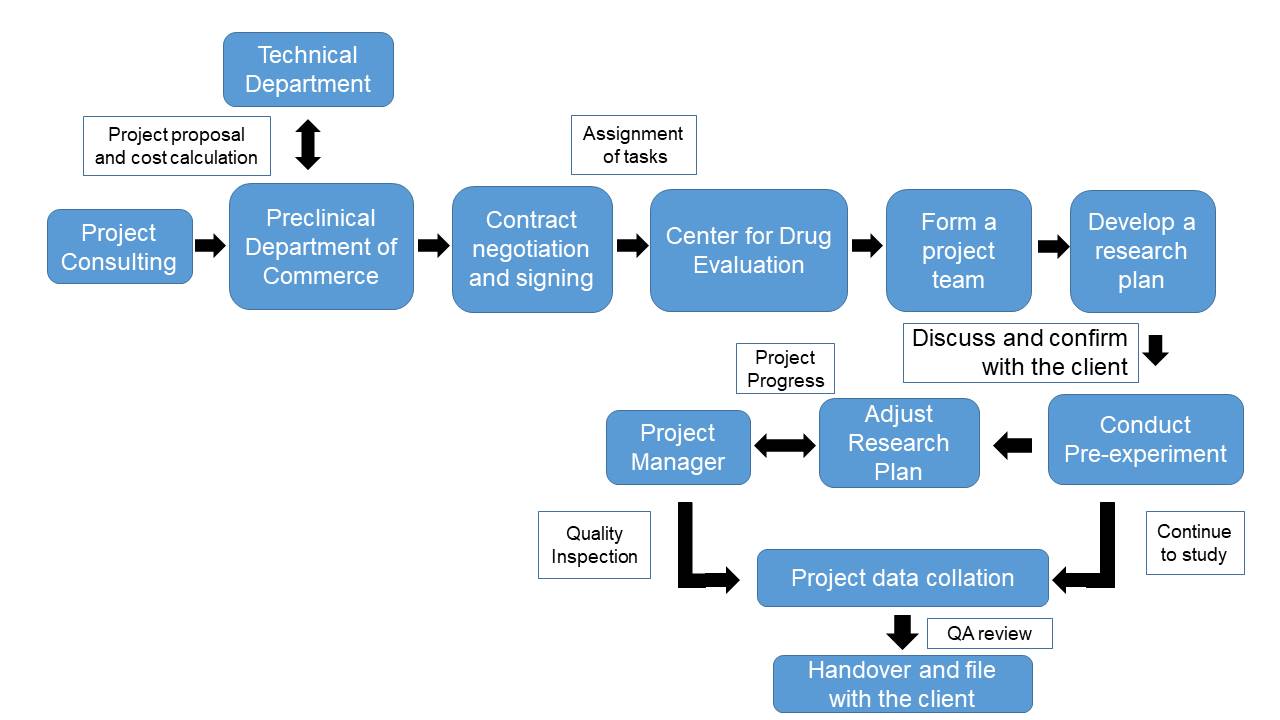
(5) Drug application and registration services
Our advantage
1.Scientific management
The center has established a complete CNA quality assurance system which ensures the timely development and effective communication during the projectmanagement. The center has a mature project operation system that compiles hundreds of standard operating procedures, covering the use of equipment and daily management, experimental operations, etc.
2.Excellent team
The center has an excellent and well-trained professional team. The department directors are professional managers with many years of preclinical research and registration experiences. The technicians have experienced a number of preclinical studies with extensive practical experiences. The center has an effective training and assessment mechanism aiming at improving our technical level. Rigorous, efficient, professional, advancing with the times and strong service awareness are the most basic guarantees forservice quality.
3.Rich experience
Through hundreds of pharmacological and toxicological services, we have accumulated a wealth of experience from more than ten years of CRO services while establishing good relationships with pharmaceutical-related government departments.
4.Excellent service
Our goals are to make the most profit for our clients, provide high-quality services, establish stable and long-term cooperative relationships with our customers and create win-win situation.
Since its establishment, the company has provided pharmaceutical research, pharmaceutical research, toxicology research and other technical services for nearly 300 customers.

Catalogue of the "New Chinese Medicine" Project underDevelopment
project name | registration category | functional indication | progress rate |
QHA Powder needle | Chinese medicine 1 | Invigorate the blood and dredge the collaterals. For the treatment of the subacute phase of ischemic cerebral infarction. This ingredient has obvious anti-platelet and antioxidant effects. | Complete pilot scale-up study and 3-month stability study |
CRN Powder needle | Chinese medicine 1 | Remove Phlegm and dredge collaterals, promote blood circulation and relieve pain; For deficiency of heart and Lung Qi, blood stasis and phlegm obstruct chronic heart failure, symptoms of palpitations, chest tightness, shortness of breath. If the symptoms are severe, it will cause cough and fatigue. | Complete pilot scale-up study |
CKZ Aerosol Inhalation Solution | Chinese medicine 2 | Indications for asthma, kidney deficiency, phlegm syndrome, As well as acute exacerbations of bronchitis and asthma, see the above symptoms. | Complete pilot scale-up study |
CHL | Chinese medicine 1 | Used to treat arrhythmias. | Complete preliminary pharmacodynamics, toxicology and pilot studies |
Saltpeter Gandan Granules | Chinese medicine 6 | It is used for chronic cholecystitis with liver-biliary damp-heat syndrome and cholangitis. | Submitted an IND notification and received an acceptance number |
Yushu Granules | Chinese medicine 6 | Sooth the liver, relieve depression, calm the mind, dryness and dampness, promote blood circulation,Indications Mild insomnia, moderate insomnia, neurasthenia and depression caused by liver qi depression. | Organize application materials and prepare to submit to IND |
Sanhua Soup | Traditional Chinese Medicine Prescription | Jiajian Xuming Soup for treatment of stroke, Sanhua Soup for constipation. | Material bench marking |
Taohong Siwu Soup | Traditional Chinese Medicine Prescription | If there are many pieces of purple, sticky blood and bruise, take Taohong Siwu Soup | Material bench marking |
Huangqi Guizhi Wuwu decoction | Traditional Chinese Medicine Prescription | 1.Deficiency of Qi and blood, Numbness of local skin 2.Nourish the body, benefit Qi and blood flow, nourish blood and remove paralysis | Material bench marking |
Baoyuan Soup | Traditional Chinese Medicine Prescription | Treatment of weak vitality, mental burnout, slow muscles, less diet, face green and white, peaceful sleep, ... Other symptoms are weak, it should be taken.. | Material bench marking |
…… | |||
Catalogue of "Chemicals" ProjectsUnder Development
Project Name | Registration Category | Indication | Progress Rate |
ASP Pills | Chemical 1 | Tonify liver and kidney, regulate blood vessels, strengthen muscles and bones. Used for the treatment of paralysis, osteoarthritis, arthritis. | Complete pilot scale-up study |
LDNF Raw Materials and Tablets | Chemical 1 | Anti - pulmonary arterial hyoertension | Complete pilot scale-up study |
CRA Raw Materials and Tablets | Chemical 1 | For heart failure with blood stasis, Myocardial ischemia; Antiplatelet, improve cardiac function, myocardial remodeling and increase blood fraction. | Complete pilot scale-up study |
OLT and its Ointment | Chemical 2 | Antiallergic drugs, Antiinflammation and antipruritic. It is used for various eczema, dermatitis, mosquito bites and other itchy skin diseases. | Complete pilot scale-up study |
Raw materials and Granules of Berberine Tannate std | Chemical 2 | This product is an anti-bacterial drug for gastro intestinal infections caused by sensitive pathogens. Medication for children. | ★Children's medicine Complete pilot scale-up study |
Clarithromycin Lactate and Powder Injection | Chemical 2 | For infections caused by clarithromycin-sensitive bacteria. | Complete pilot scale-up study |
Vardenafil Hydrochloride | Chemical 3 | Treatment of ED drugs, it works 20-30 minutes. | Complete pilot scale-up study |
Oseltamivir Phosphate and its Dry Suspension | Chemical 3 | Treatment and prevention of influenza (children's medicine) | ★Children's medicine Complete pilot scale-up study |
Voriconazole for Oral Suspension | Chemical 4 | This product is a broad-spectrum triazole antifungal drug, suitable for the treatment of fungal infections in adults and children aged 2 years and over. (Children's medicine) | ★Children's medicine Complete pilot study |
FK Liposporin Ear-Drops | Chemical 2 | For fungal ear infections, such as acute and chronic suppurative otitis media. | Complete pilot scale-up study |
DSCJ Solution for Inhalation | Chemical 2 | For bronchial asthma, asthmatic chronic bronchitis and other dyspnea caused by bronchospasm. | Complete preliminary pharmacodynamics, toxicology and pilot studies |
Riomet/Metformin Hydrochloride | Chemical 3 | Glycemic control in patients with type 2 diabetes and pediatrics. | ★Children's medicine Complete pilot study |
Memantine Hydrochloride Oral Solution | Chemical 3 | Treating medium and severe Alzheimer's Dementia | Complete pilot study |
…… | |||
Catalog of mature technology products "Chemical Solids"
Project Name | Registration Category | Functional Indication | Progress Rate |
Adefovir Dipivoxil Tablets | Chemical 4 | It is suitable for the treatment of adult chronic hepatitis B patients with hepatitis B virus and liver function compensation with continuous increase of serum amino acid transferase (ALT or AST) or liver histologically active lesions. | Passed conformance assessment |
Ibuprofen Sustained-release Capsules | Chemical 4 | It is used to relieve mild to moderate pain, and also used for fever caused by common cold or influenza. | Formal BE has passed, sorting out the application information |
Metformin Hydrochloride Sustained Release Tablets | Chemical 4 | It is used for patients with type 2 diabetes who are not satisfied with simple diet control, especially those with obesity and hyperinsulinemia. This medicine not only lowers blood sugar, but also may reduce weight and hyperinsulinemia. | Formal BE has passed, sorting out the application information |
Metformin Hydrochloride Tablets | Chemical 4 | It is used for patients with type 2 diabetes who are not satisfied with simple diet control, especially those with obesity and hyperinsulinemia. This medicine not only lowers blood sugar, but also may reduce weight and hyperinsulinemia. | Formal BE has passed, sorting out the application information |
Desloratadine Tablets | Chemical 4 | For the relief of chronic urticaria and allergic rhinitis related symptoms. | Formal BE has passed, sorting out the application information |
S-(-)-Pantoprazole Sodium Enteric-Coated Tablets | Chemical 3 | Duodenal ulcer, gastric ulcer, moderate to severe reflux esophagitis, etc. | Complete pilot test validation, in pre-BE studies |
Apixaban Tablets | Chemical 4 | Adult patients with elective hip or knee replacement to prevent venous thromboembolic events (VTE). | Complete pilot test validation, in pre-BE studies |
Trimetazidine Dihydrochloride Tablets | Chemical 4 | It is suitable for the symptomatic treatment of stable angina patients with poorly controlled or intolerable first-line antiangina therapy as an additional therapy in adults. | Formal BE has passed, sorting out the application information |
Donepezil Hydrochloride Tablets | Chemical 4 | Treatment of mild or moderate Alzheimer's dementia symptoms. | Complete pilot test validation, in pre-BE studies |
Omeprazole Enteric-coated Capsule | Chemical 3 | Applicable to gastric ulcer, duodenal ulcer, stress ulcer, reflux esophagitis and Zhuo. Moxa syndrome (gastrinoma). | Complete pilot test validation, in pre-BE studies |
Memantine Hydrochloride Tablets | Chemical 4 | Treatment of moderate to severe Alzheimer's dementia. | ★Exempt BE Complete pilot verification |
Terazosin Hydrochloride Tablets | Chemical 4 | Applicable to hypertension, can also be used alone to treat benign prostatic hyperplasia. | ★ Exempt after meal BE Complete pilot verification |
Tadalafil Raw Materials and Tablets | Chemical 3 | Treatment of ED drugs | Complete pilot verification |
Sitagliptin,Raw Materials and Chips | Chemical 4 | This product is combined with diet and exercise therapy and is used for patients with type 2 diabetes who still have poor control of blood glucose or are receiving a combination treatment of Metformin monotherapy. | Complete pilot verification |
Glucosamine Sulfate Powder | Chemical 4 | Primary and secondary osteoarthritis. | Complete pilot verification |
Montelukast Sodium Oral Granules | Chemical 4 | For the prevention and long-term treatment of asthma in children over 1 year of age. Treating aspirin-sensitive asthma patients and preventing exercise-induced bronchoconstriction. It is suitable for children aged 2 to 5 years to reduce the symptoms caused by seasonal allergic rhinitis. | Complete pilot verification |
Cefdinir Granules | Chemical 3 | Infection caused by staphylococcus, influenza bacilli and other strains that are sensitive to cefdinir. | Complete pilot verification |
Diclofenac Sodium Enteric-coated Tablets | Chemical 4 | Rheumatism and rheumatic pain, acute attacks of gout, acute mild and moderate pain. | Complete pilot verification |
Amlodipine Besylate Tablets | Chemical 4 | For hypertension and coronary heart disease. | Complete pilot verification |
…… | |||
Catalog of mature technology products "Chemical Liquid"
Project name | Registration Category | Functional Indication | Progress Rate |
Compound Aspartate,Vitamin B6 and Dipotassium Glycyrrhetate Eye Drops | Chemical 4 | For the treatment of glaucoma and ocular hypertension,as well as various conditions of increased intraocular pressure. | Production Approved |
Voriconazole for Injection | Chemical 4 | This product is a broad-spectrum triazole antifungal drug, suitable for the treatment of fungal infections in adults and children aged 2 years and over. | Organize application materials and prepare to submit registration application |
Ambroxol Hydrochloride Solution for Inhalation | Chemical 3 | This product is used for sputum thick, difficult to sputum caused by acute and chronic bronchitis. | Complete pilot test verification and 3-month stability study |
cetylcysteine Solution for Inhalation | Chemical 4 | Treatment of respiratory infections with excessive thick mucus secretions: acute bronchitis, chronic bronchitis, chronic obstructive pulmonary disease, emphysema, bronchiectasis and gummy mucus. | Complete pilot test verification |
Liraglutide and Injection | Chemical 4 | Suitable for type 2 diabetes patients to control blood sugar; suitable for patients whose blood glucose is still poorly controlled after tolerated doses of metformin or sulfonylurea drugs,and combined with metformin or sulfonylurea drugs. | Complete pilot test verification |
Doxycycline for Injection | Chemical 3 | For bacterial infections. | Complete pilot test verification |
Pantoprazole Sodium for Injection | Chemical 4 | It is suitable for acute upper gastrointestinal bleeding caused by duodenal ulcer, gastric ulcer, acute gastric mucosal lesion, and compound gastric ulcer. | Complete pilot test verification |
Olopatadine Eye Drops | Chemical 4 | Used to treat the signs and symptoms of allergic conjunctivitis. | Complete pilot test verification |
…… | |||

