

























For more questions, please click "Consult Now", a professional consultant will answer your questions.








Boji provides comprehensive and high-coverage third-party auditing services to domestic and foreign clients including, but not limited to:
Clinical trial site audit
• Phase I-IV clinical trials and post-market re-evaluation
• Drugs, medical devices, diagnostic reagents, bioequivalence, pharmacokinetic clinical trials, etc.
• Management and detection of biological samples (eg. PK and BE)
Medicine clinical trial facility evaluation
• Evaluation of the clinical trial institution’s quality management system
• Assessment of resources, facilities and implementation capacity of clinical trial institutions to conduct trials
Supplier audit
• Examples:central laboratories, pharmaceutical logistics providers, IWRS/IVRS, EDC, statistical agencies, CRO, SMO, etc.
• Supplier qualification audits, compliance audits, etc.
Foreign Drug Administration / NMPA inspection, pre-verification Audit
Research Document (Trial Master File=TMF) Audit
Audit of Data Management and Statistics
Since its establishment, the company has provided pharmaceutical research, pharmaceutical research, toxicology research and other technical services for nearly 300 customers. 
Professional audit team
Deputy General Manager Meng Lili is a well-known domestic expert in clinical trial quality management, responsible for the management of the audit team and audit technical services. She has been a lecturer in the NMPA (formerly CFDA) inspector training class, and was the founder and CEO of adomestic audit company. She has been a speaker at various domestic academic conferences throughout the year and accurately grasped the main points and trends of clinical trial audits.
The auditor team has an average of more than 8 years of industry experience, a maximum of 19 years of management experience and audit experience in the field of clinical trials.
We have an audit consultant team in various fields of clinical trials. And audit services in special areas have solid technical guarantee.
Rich auditing practice
Establish a clinical trial audit data and quality problem database, regularly analyze trends of quality problem, effectively grasp risk signals and provide big data support and guidance for audit activities.
With more than 400 audits, we have rich practical experience in auditing and our audit evaluation is more targeted and professional.
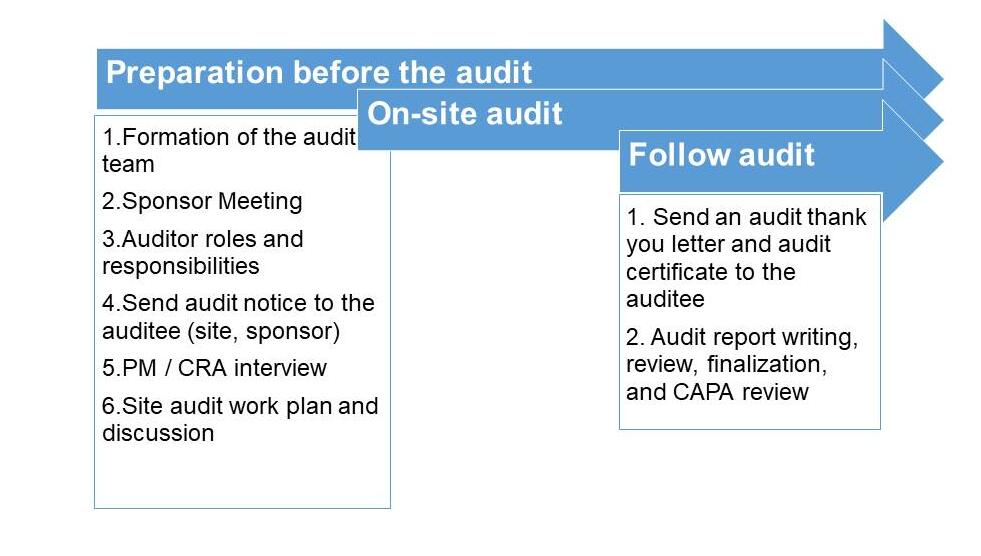
Professional audit technical services
We have a complete audit service process and professional technical services, which can fully ensure the professionalism of the audit. With high customer satisfaction, it also helps to maintain the brand image of the sponsor.
With deep auditing skills and unlimited to the discovery of process and routine problems, we are good at mining systemic problems and deep hidden problems.
The findings of the audit are not superficial, but they are described systematically and comprehensively through the basis of the audit, objective issues, audit evidence and negative effects.
With regard to the key issues found in the summary and systematic analysis of audit, we can put forward effective systematic improvement suggestions and action plans to help companies learn from external experiences, break through the fixed thinking mode of the enterprise and optimize and improve their own systems.

