

























For more questions, please click "Consult Now", a professional consultant will answer your questions.








Drug clinical research refers to the systematic study of drugs in humans (patients or healthy volunteers) to confirm or reveal its effects, adverse reactions, and / or absorption, distribution, metabolism and excretion laws. The purpose is to determine the efficacy and safety of the trial drugs, mainly including Phase I-IV clinical studies. According to the laws and regulations of the registration of new drugs in China, drugs clinical research must be conducted by medical institutions qualified as clinical trial institutions. Our clinical research service is accepting the commission of the sponsor, formulating a clinical research plan, monitoring the clinical research process, conducting clinical trial data management and assisting in completing the clinical research summary report with the sponsor and the main investigators. At the same time, the company also undertakes external consulting services related to new drug research and development, such as data management, statistical analysis and agency registration. Our company also independently undertakes other consulting services related to new drug research, such as data management, statistical analysis and agent registration.

In the field of clinical research services, until September 30th, 2019, our company has provided more than 400 customers, with more than 400 preclinical research services and more than 800 clinical research services, which basically cover all professional fields of drug therapy and include more than 60 innovative drugs with higher difficulty. We help customers obtain more than 60 new drug certificates and about 80 production approvals. The completed clinical research service projects cover avarieties of professional fields of medical treatment such as cardiovascular, respiratory, digestive, endocrine, urinary, oncology, neurology, gynecology, dermatology, otolaryngology, ophthalmology, pediatrics, surgery and anesthesia.
Our advantage:
1. Keen policy insight
We have smooth consultation and communication channels with relevant departments such as NMPA (formerly CFDA), Development and Reform Commission, Health and Health Commission, Ministry of Human Security.
With sharp perspective, we can closely integrate future policy guidance in evaluation design and implementation and meet the policy needs.
We are familiar with the latest policy changes such as R & D, medical insurance, essential medicines, and pricing.
2. Comprehensive experts team
We have a talent team covering various professional fields of medicine, pharmacy, epidemiology, statistics, pharmacoeconomics, marketing, business management, law, and public management. We can develop an overall plan that fits corporate strategy, considering from multiple perspectives such as corporate strategy, marketing, product line planning and product clinical positioning.
3. Rich expert resources
We have extensive cooperation with opinion leaders and authoritative academic institutions in various therapeutic fields in China, such as clinical expertsin the Chinese Medical Association, Chinese Pharmaceutical Association, Chinese Academy of Chinese Medicine, China Association of Chinese Medicine, universities and pharmaceutical administration departments. We can organize domestic authoritative experts to jointly customize clinical protocols. After completion, the paper will be published in influential academic journals abroad to expand domestic academic influence, improve brand image and promote sales.
4. Extensive cooperative hospitals
There are more than 800 cooperative hospitals distributed across the country.
5. A well-distributed office network—in Guangzhou Headquarters, Beijing Subsidiary, Shanghai Subsidiary and 25 Provincial Capital Cities
6. A huge subject database and perfect subject recruitment system
7. Advanced data management software — EDC electronic case collection system
8. Rich experience in innovative drug projects (more than 60 projects)
Especially in the field of anti-infection, liver disease, digestion, tumor, rheumatism and orthopedics, we have accumulated rich experience in project operation and management.
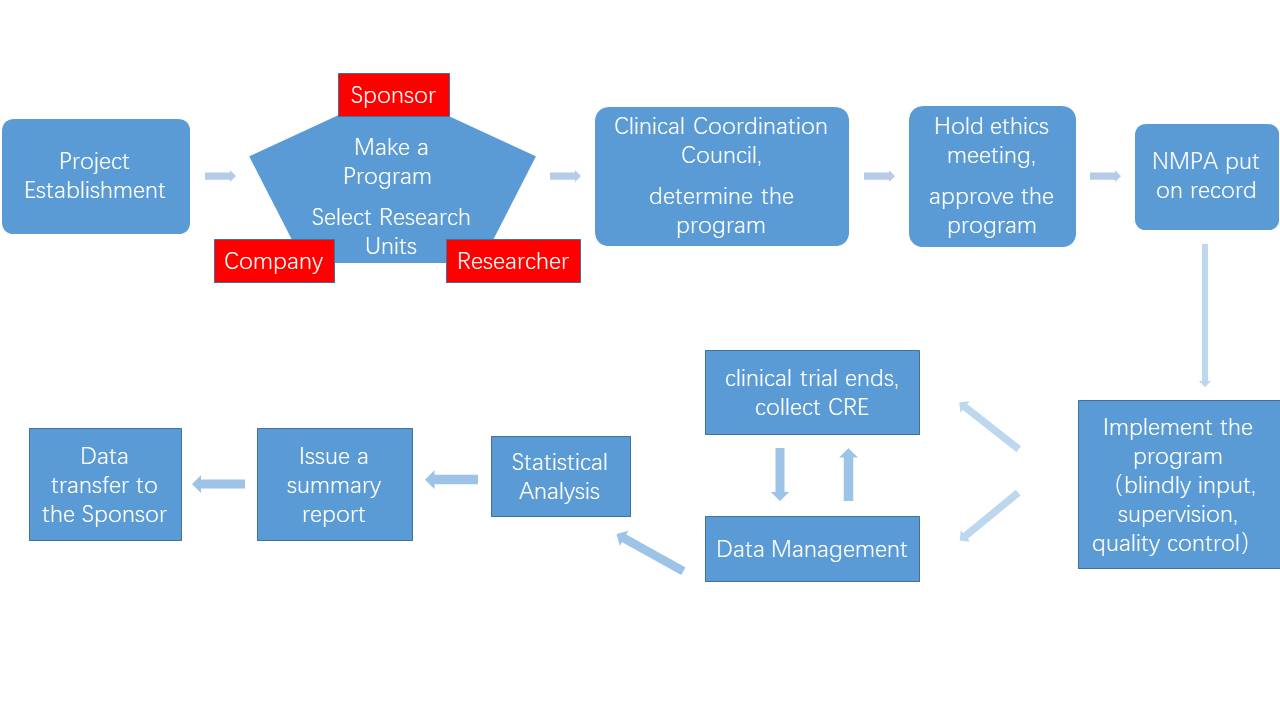
The main processes of clinical research services are as follows:
(1)Set up project
After the project successfully signed acooperation agreement with the sponsor, business personnel will establish the project with company's clinical department, medical department and data management statistical analysis and organize a meeting to clarify the scope of the project and the needs of the sponsor.
(2)Develop draft plan and select research units
The company's clinical department is responsible for selecting clinical research units (detailed investigation and evaluation of each research unit), one of which is the team leader unit (individual sponsors will recommend a research unit as the team leader unit) and contacting the participating units.
(3)Hold clinical research coordination meeting
The company, sponsor and all research units hold a clinical research coordination meeting to discuss and determine the clinical research plan.
(4)Approved by the ethics committee and put on record
After confirming the clinical research plan, it is reported to the ethics committee of the team leader for approval. The unit team leader convenes the ethics committee to approve the clinical research plan and other materials. After obtaining the approval, the sponsor submits the research plan to the National Medical Products Administration and the relevant Provincial Medical Products Administration for registration and record. If the ethics committee of the team leader makes suggestions for revision, the ethics committee needs to be convened again after the plan and the other materials should have been modified before it can be implemented. Except for the unit team leader, other participating units will hold sub-center ethics review meetings as needed to review and approve the research plan and other materials. If the participating units raise objections, they need to give feedback to the unit teamleader and the sponsor to discuss and modify the plan again. If the participating units have objections to the final clinical research plan and other materials, they can choose to exit the program. After the clinical plan is determined, the research materials of the unit team leader including the ethics committee approval, clinical research protocol, and drug test report need to be recorded in all participating units.
After obtaining the approval of the ethics committee, non-participating statistical professional staff will randomly blind compile the clinical trial drugs provided by the sponsor. Sponsors with a foreign investment background need to obtain approval from the ethics committee. The trial can only be carried out after approval.
(5)Clinical trial start, run, and end
Before the clinical research begins, the clinical department signs the clinical research agreement with all clinical research units. After the clinical research agreement is signed, the sponsor is notified to send the clinical trial drugs to the corresponding research unit. The project manager and inspector in charge of the project train relevant researchers on clinical research protocols and the trial officially starts. During the clinical research, the inspector strictly complies with the relevant regulations to check whether the enrolled cases meet the selection requirements and exclusion criteria stipulated in the scheme, the research data were filled in accurately, timely and truthfully, the laboratory data were checked and verified, and an audit report was issued. After the patient leaves the group, the inspector checks the case data of all research centers, recovers the research data and the remaining clinical trial drugs, and counts the remaining clinical trial drugs for the sponsor.
(6)Data management and statistical analysis
After the clinical research data is recovered, the data administrator formulates a data management plan, compiles a data verification plan according to the clinical trial plan, checks the data, issues a questionnaire to the investigator to answer questions and corrects the database based on the investigator's answer sheet. After answering the questions, a data review meeting will be held, and the database will be locked after the meeting (unblinded trials will be performed during blind tests). Based on the locked database, statisticians perform statistical analysis in accordance with the plan and issue a statistical analysis report.
(7)Summary meeting, summary report
After the statistical analysis report is completed, the company's business units (including the medical department, clinical department, statistical analysts) and the research units hold a clinical research summary meeting to discuss and finalize the statistic alanalysis report, and summarize the clinical research. Ministry of Medicine prepares a summary report based on the statistical analysis report. Each unit reports the draft and sends to each research unit for review andfinalization, signature confirmation and finally submit to each drug clinical trial institution for signature. The clinical research summary report and other research materials are important materials for the sponsor to apply for a new drug certificate and drug registration approval.
(8)Quality control, data transfer
The entire process of the project is subject to quality management and control. Before the project begins, the quality management requirements of the project are clearly defined with the support of the quality control department. During the project, the sample is systematically and regularly examined. Before submitting to the relevant departments, the application materials will be fully approved by the quality control department to ensure their quality. After the test is completed, the materials that have finally passed the quality control will be uniformly transferred to the sponsor and the transfer list signed.
Since its establishment, the company has provided pharmaceutical research, pharmaceutical research, toxicology research and other technical services for nearly 400 customers.

1. Hydrogen and Oxygen Atomizer—a medical device for the adjuvant treatment of COVID-19 (led byZhong Nanshan, Academician of Chinese Academy of Engineering)
2. TPN729—Category 1 of Chemical Drugs, for the treatment of Erectile Dysfunction (ED)
3. Category 1 of Chemical Drugs, for the treatment of Hepatitis B
4. HEmay020 Capsules—Category 1 of Chemical Drugs, for the treatment of Non-Small Cell Lung Cancer (NSCLC)
5. YPS345 Tablets—Category 1.1 of Chemical Drugs, for the treatment of Respiratory Diseases
6. EPO Fusion Protein—Category 1 of Therapeutic Biological Products, for the treatment of Renal Anemia
7. SK08—Category 1 of Therapeutic Biological Products, for the treatment of Irritable Bowel Syndrome (IBS)
8.Piper Phentonamine Hydrochloride—Category 1 of Chemical Drugs, for the treatment of Acute Heart Failure
9. Xiongdin Afilcitrate—Category 1 of Chemical Drugs, for the treatment of Erectile Dysfunction(ED);
10. MRX-Itablets—Category 1 of Chemical Drugs, for the treatment of Skin Infections;
11. L-phencynonate Hydrochloride Tablets—Category 1 of Chemical Drugs, for the treatment of Motion Sickness;
12. Ginkgolide B for Injection—Category 1 of Traditional Chinese Medicines, for the treatment of Acute Ischemic Stroke;
13. Pre-IND Application: 765IGF-MTX—An innovative drug intends to conduct international multi-center clinical trials (Hyperosteogeny Syndrome)
14. Pre-IND application: YJ001 for spray—Category 1 of Chemical Drugs, for the treatment of Diabetic Peripheral Neuralgia in Zhejiang;
15. Pre-IND application: Interferon (Orphan Drug)—Category 1 of Chemical Drugs;
16. .Pre-IND application: SI-006— Category 1 of Therapeutic Biological Products;
17. Imported Drug Registration: Masetinib mesylate tablets, for the treatment of prostate cancer, melanoma and pancreatic cancer;
18. Artificial Heart—Category Ⅲ of Medical Devices, for the treatment of Refractory End-stage Heart Failure;
19. Left Atrial Appendage Occluder System—Category Ⅲ of Medical Devices, for the treatment of Nonvalvular atrial fibrillation (led by Ge Junbo, Academician of the Chinese Academy of Sciences).

